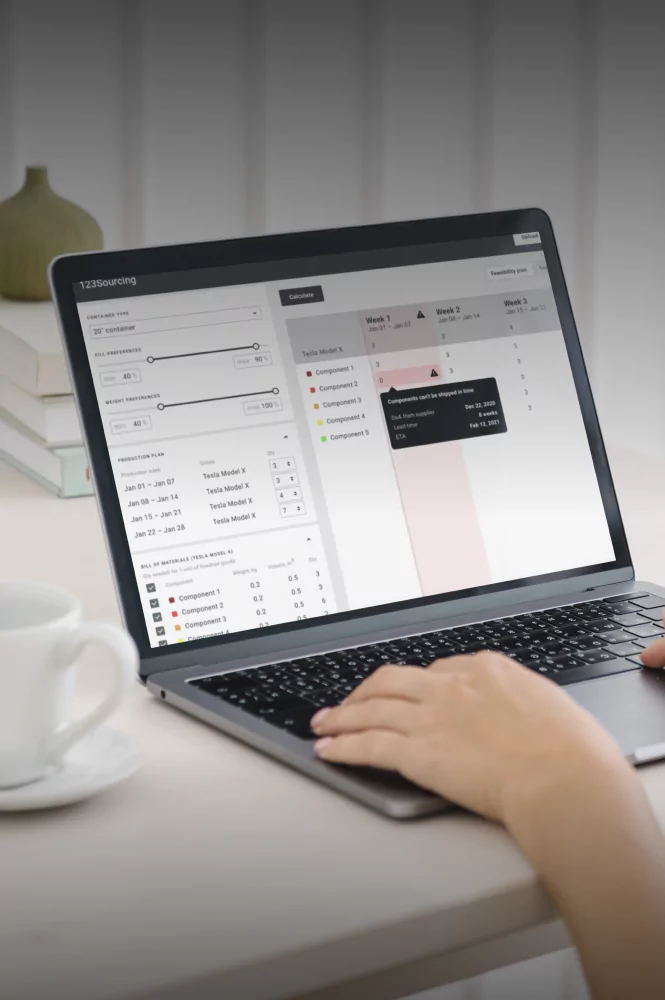
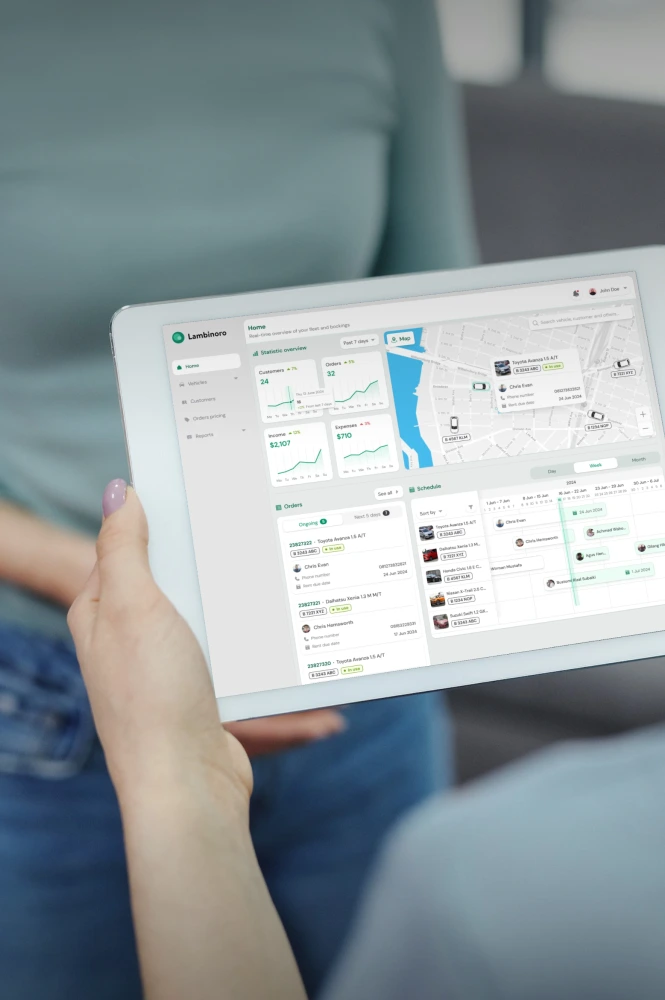
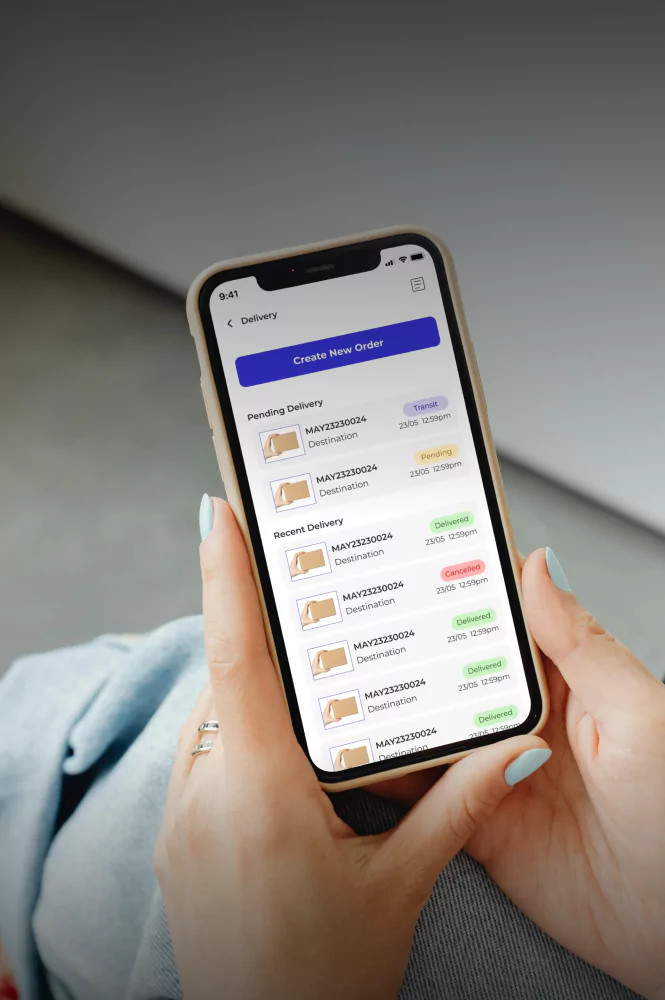
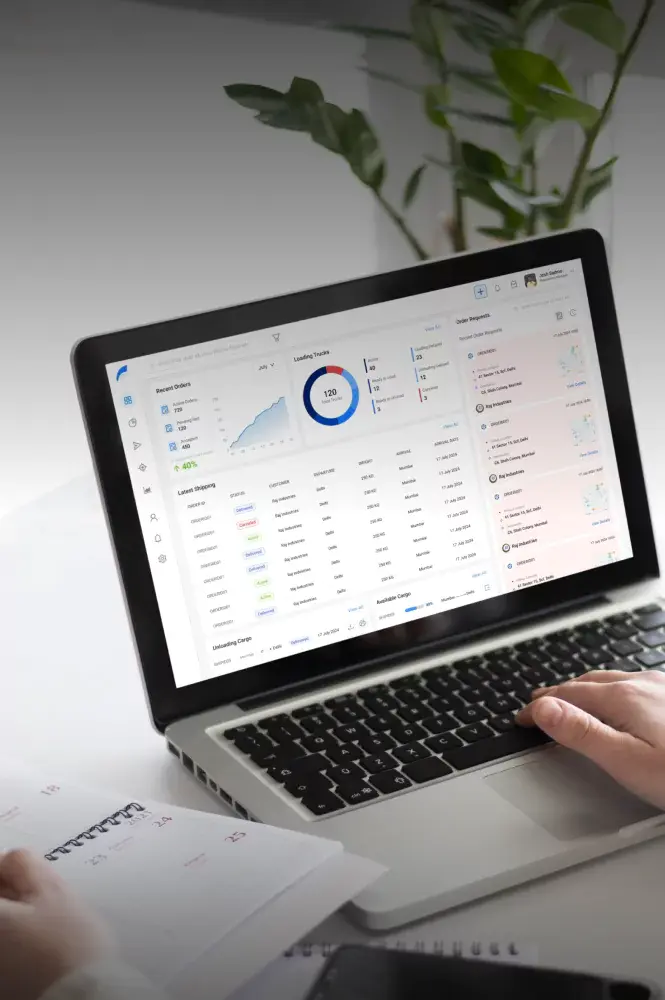
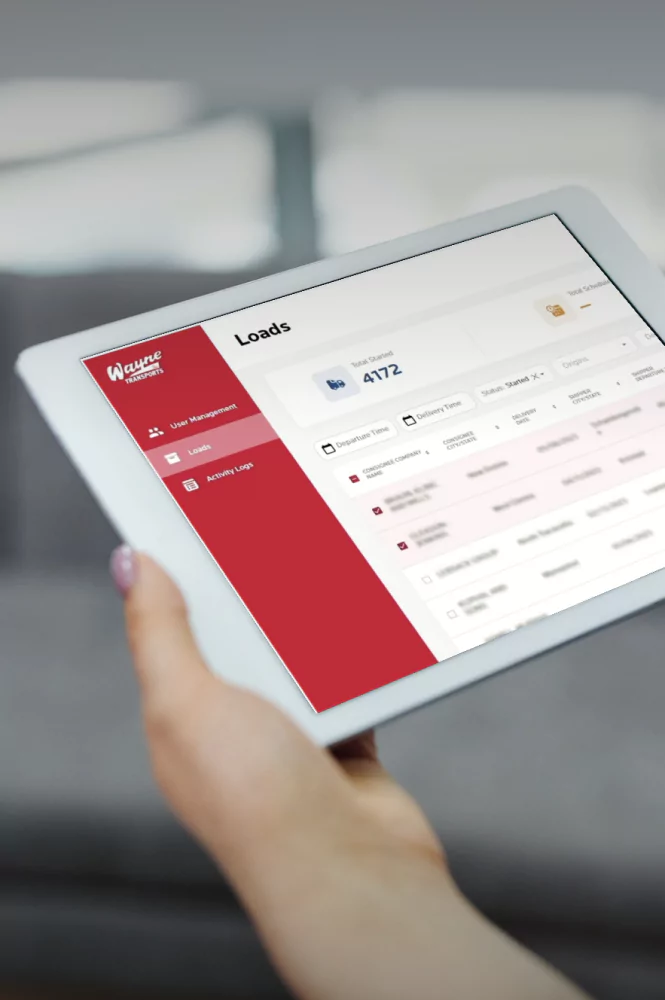
Cold Chain Software & Hardware Development Services
Yalantis delivers full-cycle, GxP-validated cold chain technology for 3PLs and cold chain providers. We build integrated software and IoT hardware solutions that provide the unalterable scientific evidence required for FDA, EMA, and GxP compliance.
Challenges Our Cold Chain Technology Expertise Solve
-
Catastrophic product loss from temperature excursions
Instead of discovering a costly temperature breach after delivery, our solutions provide real-time alerts. This transforms your process from reactive damage control to proactive intervention, saving multi-million dollar shipments before they are lost.
-
FDA/EMA audit failures due to incomplete or alterable data
Forget manual logs and disparate CSV files. We build 21 CFR Part 11 compliant systems with unalterable, time-stamped audit trails. This provides the single, validated source of truth that satisfies regulatory inspectors and defends your release decisions.
-
Data silos between carriers, 3PLs, and pharma clients
Our platforms are built for integration, breaking down walls between your WMS, QMS, and your clients’ systems. We provide a unified data layer, giving all stakeholders real-time visibility and a shared, unalterable record of the product’s journey.
-
High operational costs from manual QA processes
Automate the review of temperature data. Our systems automatically flag excursions, correlate data from all loggers, and generate audit-ready reports, reducing manual QA overhead by up to 90% and freeing your team to manage exceptions.
-
Lack of trust in hardware and data integrity
Stop relying on consumer-grade loggers. We design and deploy GxP-compliant, NIST-traceable IoT sensors with validated firmware, ensuring the data you collect is scientifically accurate and legally defensible from the moment it’s recorded.
-
Inability to perform proactive risk management
Without aggregated data, you can’t identify high-risk lanes, poor-performing carriers, or inadequate packaging. Our platforms provide the analytics to move beyond simple monitoring to active lane validation and risk mitigation.
Our Cold Chain Technology Development Services
-
End-to-End Validated Cold Chain Platform Development
A complete, turnkey solution for 3PLs and pharma companies. We design, build, validate, and deploy a GxP-compliant cloud platform that serves as your central compliance hub and single source of truth for all temperature-controlled assets.
Key Features:
- GxP-Validated Cloud Platform
A fully validated environment built on GAMP 5 principles, ready for your VMP, IQ, OQ, and PQ, ensuring it meets the most rigorous FDA and EMA standards. - Centralized Monitoring Dashboard
A real-time command center providing live visibility of all active shipments, storage units, and their environmental conditions, accessible by your team and your clients. - Product Profile & Alerting Engine
A sophisticated rules engine that allows you to set specific, multi-stage temperature profiles and alert rules for each unique product or SKU. - Unalterable Digital Record (ALCOA+)
Every shipment’s data is captured in a time-stamped log that adheres to ALCOA+ data integrity principles (Attributable, Legible, Contemporaneous, Original, Accurate). - Automated Audit & Compliance Reporting
One-click generation of audit-ready Temperature Journey Reports, Excursion Summaries, and Chain of Custody logs, drastically reducing QA and compliance burdens.
- GxP-Validated Cloud Platform
-
Custom IoT Sensor & GxP-Firmware Engineering
A specialized hardware engineering service for when off-the-shelf loggers don’t meet your needs. We design or pick custom-calibrated environmental sensors (both single-use and reusable) with secure, unalterable GxP-compliant firmware.
Key Service Deliverables:
- NIST-Traceable Sensor Design
Development of hardware with certified and calibrated temperature, humidity, light, and shock sensors traceable to a National Institute of Standards and Technology (NIST) reference. - GxP-Compliant Firmware
Secure, locked-down firmware that prevents data tampering, ensures reliable connectivity, and guarantees data gaps are not created or concealed. - Custom Form-Factor & Battery Life
Hardware designed for your specific use case, whether it’s for cryogenic shippers, reusable pallets, or full-truckload monitoring with multi-year battery life. - Secure Data Transmission & Storage
End-to-end encryption from the device to the cloud, ensuring data is secure and protected both in-transit and at-rest.
- NIST-Traceable Sensor Design
-
Cold Chain Temperature Monitoring Devices
A specialized hardware service for when off-the-shelf loggers fail to meet your GxP or operational needs. We design, validate, and deploy custom cold chain temperature monitoring devices tailored precisely to your high-value assets and supply chain.</p>
Key Service Deliverables:
- NIST-Traceable & Calibrated Sensors
We deploy certified, high-precision temperature and humidity sensors with NIST-traceable calibration, ensuring your data is scientifically accurate and audit-proof. - Secure GxP-Compliant Firmware
Our devices run validated, locked-down firmware that prevents tampering and creates unalterable, ALCOA-compliant data records from the point of capture. - Custom Form-Factors & Use Cases
We engineer hardware for your exact needs, including custom enclosures, multi-year battery life, and sensors qualified for extreme cryogenic environments. - Reliable Data Connectivity
We configure reliable connectivity to ensure no data gaps, automating the secure flow of sensor data from the device to your cloud platform. - Full Hardware Validation (IQ/OQ)
We deliver a complete validation package, including IQ/OQ, to formally document that the devices are qualified and fit for their intended GxP use.
- NIST-Traceable & Calibrated Sensors
-
QMS, ERP, and WMS Integration Services for Pharma
A data engineering service designed to break down information silos. We build robust, two-way integrations that connect your cold chain platform to the core systems that run your business and your clients’ operations.
Key Service Deliverables:
- QMS Integration (Veeva, TrackWise)
Automatically trigger quality events, CAPAs, and deviations in your Quality Management System based on real-time temperature excursions. - ERP & WMS Integration (SAP, Oracle, Manhattan)
Seamlessly associate shipment data with purchase orders, batch numbers, and warehouse locations. Automate inventory release or hold decisions in your WMS/ERP. - Carrier & Telematics API Aggregation
Pull in data from carrier APIs and in-vehicle telematics to create a single, enriched record that layers location and asset data with environmental data. - Client Data-Sharing Portals
Build secure, branded portals that give your pharmaceutical clients self-service access to the real-time status and compliance reports for their specific shipments.
- QMS Integration (Veeva, TrackWise)
-
Cold Chain Data Analytics & Lane Validation Module
A standalone data science module that transforms your historical data into actionable intelligence. This module helps you move from reactive monitoring to proactive risk management, optimizing your entire cold chain network.
Key Service Deliverables:
- Lane Risk Assessment Dashboards
Interactive tools that analyze historical excursion data to identify high-risk shipping lanes, carriers, or packaging configurations that require corrective action. - Proactive Excursion Warning Models
AI models that analyze real-time data trends (e.g., subtle temperature drift) to predict a likely excursion before it happens, enabling proactive intervention. - Thermal Packaging Performance Analysis
Analyze data to compare the real-world performance of different thermal shippers and packaging solutions, enabling data-driven procurement decisions. - Carrier & 3PL Performance Scorecards
Generate automated reports that score your logistics partners on their cold chain performance, providing objective data for QBRs and contract negotiations.
- Lane Risk Assessment Dashboards
Cold Chain Solutions for Your Sector
Case studies
Benefits of Building Your Cold Chain Solution With Us
-
Ensure Unwavering Regulatory Compliance
Our expertise in GAMP 5 and 21 CFR Part 11 is built into our DNA. We deliver the validated, audit-proof record you need to satisfy FDA and EMA inspections and protect your license to operate.
-
Prevent Catastrophic Product Loss
Our integrated hardware and real-time alert engines empower you to intervene before a temperature excursion becomes a multi-million dollar product loss, protecting your bottom line.
-
Protect Brand Reputation & Patient Safety
We provide the unalterable scientific evidence to prove your product’s complete temperature journey, ensuring the safety of every patient and consumer and protecting your brand from a quality-related recall.
-
Eliminate Data Silos with a Single Source of Truth
Our integration-first approach connects your hardware, software, and partners into one validated platform, creating a single, unalterable source of truth for all stakeholders.
-
Reduce Manual QA & Operational Overheads
We automate the entire compliance workflow—from data collection to report generation—allowing your QA team to stop chasing paperwork and start focusing on high-value exceptions.
-
Unlock Actionable Asset Intelligence
We transform raw temperature data into a strategic asset. Our analytics help you identify high-risk lanes, optimize packaging, and enforce carrier performance, driving continuous improvement.
Technologies we work with
-
Rust
-
C
-
C++
-
Kotlin
-
Bootloader
-
Linux Kernel
-
AWS IoT
-
Arduino
-
ESP32
-
STM32
-
NRF52
-
Zephyr
-
LoRaWAN
-
MQTT
Compliance and security support
Security for your supply chain
-
ISO 28000
-
DTLF
-
CISA / NIST
RFID-powered Logistics
-
GS1 EPC/RFID
-
ISO 17363–17365
Testimonials from our clients
Predictive Maintenance ServicesInsights

How Cold Chain IoT Solutions Ensure Pharmaceutical Shipment Safety and Compliance?
Discover how cold chain IoT solutions use real‑time monitoring, data analytics, and compliance controls to ensure the safety and regulatory adherence of perishable goods throughout the supply chain.
Visualizing supply chain data with logistics dashboards
Learn about different supply chain analytics dashboards and supply chain visibility tools that can help you resolve issues that stop your company from growing.

How to Manage Pharmaceutical Labeling for Temperature-Sensitive Shipment
Overcoming the limitations of paper labels in cold chain logistics with robust, updatable digital displays for temperature-sensitive pharmaceuticals.
FAQ
How do you ensure your cold chain monitoring solutions are GxP-compliant and audit-proof?
We don’t just sell software; we deliver a fully validated solution. We use the GAMP 5 framework to provide documented, objective evidence (IQ/OQ/PQ) that your entire cold chain monitoring process is tested, controlled, and ready to face an FDA or EMA inspection. This de-risks your audits.
What is the business case for switching from disposable loggers to your active cold chain temperature monitoring devices?
Disposable loggers only confirm a product loss after it happens. Our active platform is a proactive, ROI-generating tool. Our cold chain temperature monitoring devices send real-time alerts, allowing you to intervene before a loss occurs, saving high-value assets and automating QA release decisions.
How do your cold chain tracking and monitoring solutions integrate with our WMS, ERP, or QMS?
Our solutions are integration-first. We are technology partners who build robust, two-way APIs to connect our platform with your core systems like SAP, Oracle, or Veeva. This automation turns monitoring data into action, such as triggering a quality event in your QMS or placing a batch on hold in your WMS.
How do your cold chain monitoring solutions meet FDA 21 CFR Part 11 and ALCOA+ data integrity requirements?
We ensure your data is 100% defensible. Our platforms are architected for 21 CFR Part 11, providing unalterable electronic records that meet ALCOA+ standards. All data is time-stamped, secure, and linked to a full audit trail with compliant electronic signatures for GxP-critical release decisions.
What is the end-to-end process for deploying and validating your cold chain monitoring platform?
Our GAMP 5-aligned service manages all complexity. We start with a User Requirements Specification (URS) to map your needs, then write the Validation Master Plan. Our quality team executes the full IQ/OQ/
Can your system monitor both in-transit shipments and our GxP cold chain storage?
Yes, our platform unifies your entire operation. We provide a single, GxP-validated solution for real-time cold chain storage temperature monitoring (freezers, cold rooms) alongside your in-transit shipments. This eliminates data silos and provides one compliant, audit-proof record for your entire chain of custody.
Contact us

got it!
Keep an eye on your inbox. We’ll be in touch shortly
Meanwhile, you can explore our hottest case studies and read
client feedback on Clutch.

Nick Orlov
IoT advisor
How to get started with IoT development
-
Get on a call with our Internet of Things product design experts.
-
Tell us about your current challenges and ideas.
-
We’ll prepare a detailed estimate and a business offer.
-
If everything works for you, we start achieving your goals!