Check this out:
- In 2019, AliveCor released its KardiaMobile® 6L – the world’s first available six-lead personal ECG device, cleared by the Food and Drug Administration (FDA). It’s a mobile ECG device for detecting the presence of atrial fibrillation (AFib), a heart rhythm irregularity, that can increase the risk of stroke.
- In 2020, Abbott Laboratories’ FreeStyle Libre 3 received a CE mark in Europe and, in 2022, a clearance from FDA in the USA, which officially made it the thinnest and most accurate 14-day glucose sensor that doesn’t need fingersticks for calibration.
- In 2022, FDA cleared Dexcom G7, a continuous glucose monitoring (CGM) system, which allows diabetics to track their blood sugar levels in real-time and doesn’t require a separate receiver.
Do you know what unites them all?
It’s not only improving the quality of life for patients who previously couldn’t manage their conditions. These advancements also propel a massive and highly promising industry and IoT medical devices market towards innovative approaches to treating serious chronic diseases. In other words, they push the need for smarter IoT healthcare solutions.
In short, these advancements make Medical Internet of Things (MIoT) devices, or simply medical IoT devices, as accessible and user-friendly as smartphones and wearables, leading to a surge in demand. More importantly, they are FDA-approved, ensuring safety and accuracy for medical use. Beyond improving quality of life and self-monitoring, these smart devices empower clinicians to tailor and improve treatment plans based on the valuable data they collect.
This undoubtedly sounds like a dream come true for many patients seeking greater freedom, stability, and confidence in daily life. However, the journey from vision to reality, encompassing prototyping, FDA approval, and finally, release, is a complex one. Let’s delve into the key stages involved:
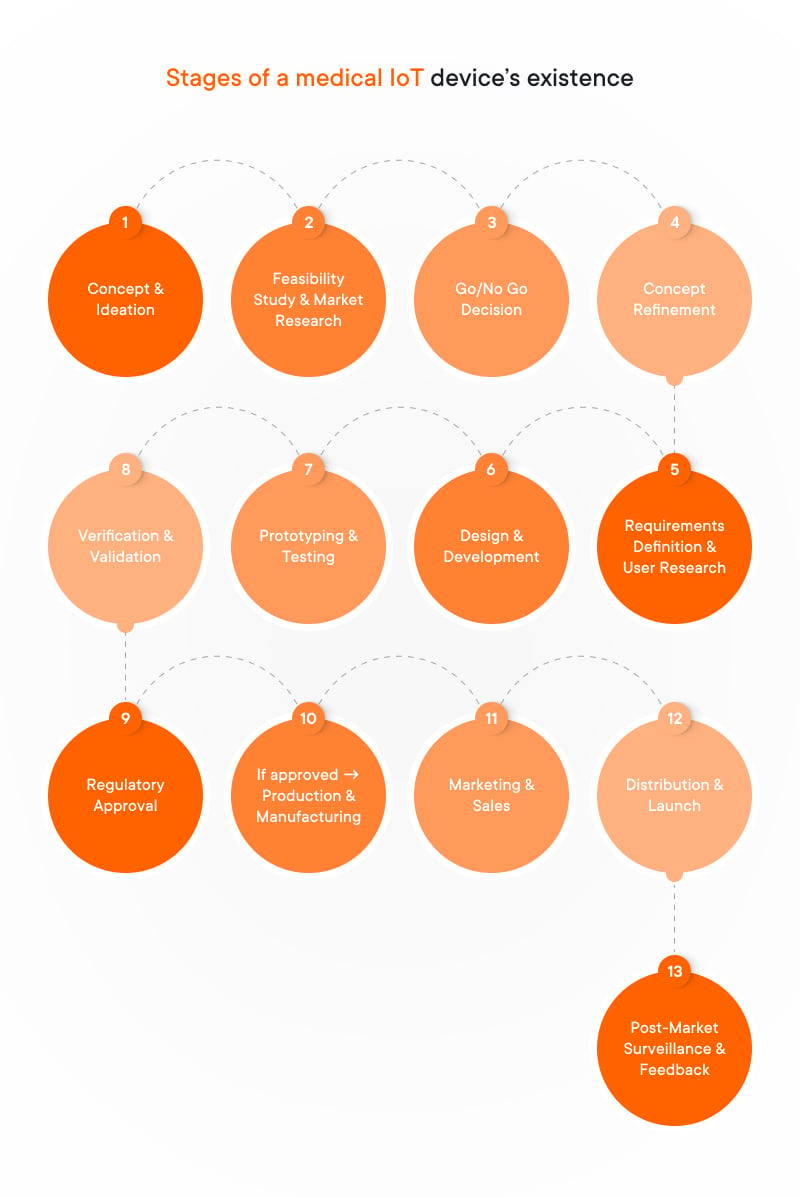
In this article, we’ll specifically focus on the three most important stages of sensor integration:
- State of the medical IoT value proposition
- Prototyping
- Testing
- Compliance
But first, let’s get a bit more informed about the state of the medical IoT value proposition.
Interested in medical device development services?
We design and develop Class II and III medical devices from concept to certification, helping you reduce post-market costs by 30%.
Types of medical IoT devices and sensors that comprise the MIoT universe
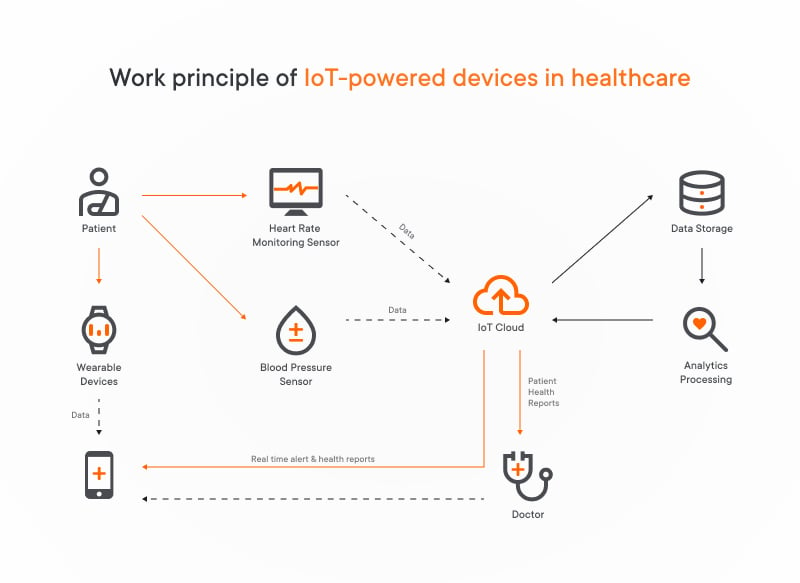
We won’t waste your time on explaining how IoT in medical devices works. If you somehow forgot, we offer you quickly get through this scheme and remember the basics, this will be quite enough:
Now, let’s get into more interesting things: how far has the MIoT industry gone?
First, we have to clarify that it’s not only purely medical IoT devices that are used in the healthcare industry. Our wearables like smartwatches can also be used to get more detailed information about a patient’s health. The only difference is that their data won’t be considered as seriously as from certified internet of Things medical devices.
Data about our physical activity, heart rate, and other vitals can serve as an additional source of information that just enriches the context for healthcare providers while treating and monitoring patients as well as diagnosing them. These types of devices are much more affordable than IoT medical devices, so that’s why their greater widespread and accessibility make them a big attention point in the overall treatment process.
Incorporating IoT services into the MIoT ecosystem enhances the ability to monitor patient health effectively and provides healthcare professionals with real-time data for better decision-making. This integration not only improves patient outcomes but also streamlines operations within healthcare facilities, making it a crucial aspect of modern medical practices.
So, what about the overall classification?
Medical IoT devices break into the following categories:
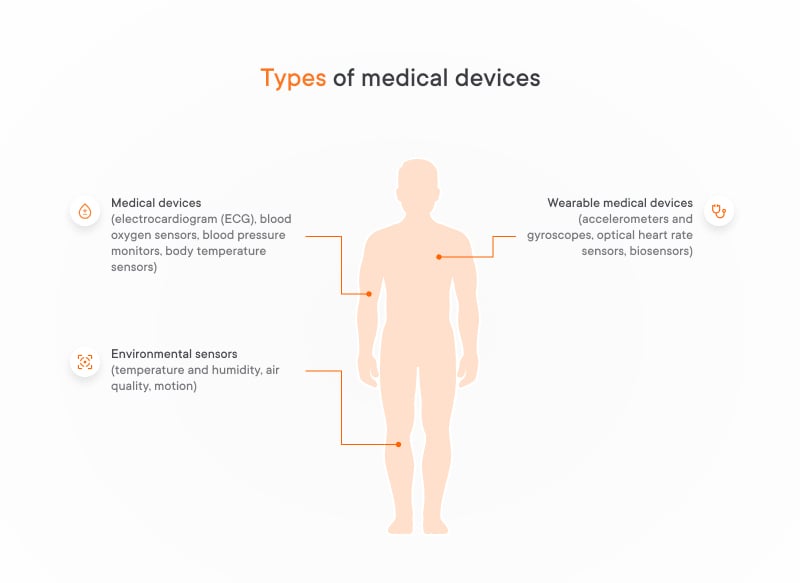
Medical devices: IoT as a driving force for 24/7 measuring of vital signs
IoT and medical devices: IoT as a driving force for 24/7 measuring of vital signs.
Medical IoT devices are becoming increasingly common. These devices use small sensors to measure a patient’s vital signs and provide continuous data, empowering individuals and healthcare providers to make informed decisions about health. Such IoT-enabled medical devices include:
- Electrocardiogram (ECG) that monitors the electrical activity of the heart, crucial for detecting arrhythmias and other heart conditions. It can capture heart rate characteristics 24/7, with IoT data visualization and notification enabling timely alerts for abnormal patterns.
- Blood oxygen sensor that measures blood oxygen saturation, vital for respiratory assessments and monitoring sleep apnea. The spread of COVID-19 highlighted the importance of monitoring blood oxygen levels.
- Blood pressure monitors that help to observe systolic and diastolic blood pressure, aiding in hypertension management as well as preventing and managing heart disease.
- Body temperature sensor that quickly identifies fevers and tracks temperature changes, important for infection control and early disease diagnosis.
Innovation in IoT: wearable medical devices
Wearable medical devices have become increasingly popular, offering a convenient way to monitor our health and fitness. These devices rely on a variety of sensors to collect data about our bodies. Here’s a closer look at some of the most common vital signs monitoring devices and what they do:
Accelerometers and gyroscopes:
- Track movement patterns, steps taken, and sleep quality
- Help assess physical activity levels and identify potential sleep disorders
Optical heart rate IoT medical sensors:
- Use light technology to measure heart rate non-invasively
- Ideal for continuous monitoring during exercise or daily activities
Biosensors:
- Analyze bodily fluids like sweat or tears for various biomarkers
- Allow for non-invasive monitoring of glucose levels, hydration, and even stress levels
IoT medical devices: Examples of environmental sensors
Environmental sensors collect data about a patient’s surroundings, helping to create a safe and healthy environment. As part of IoT solutions, these devices play a crucial role in healthcare. Here’s a closer look at some of examples of IoT medical devices:
- Temperature and humidity sensors that monitor environmental conditions in hospitals or patient homes. They ensure optimal comfort for patients and minimize infection risks.
- Air quality sensors that detect pollutants and allergens in the environment. They are of great help when it comes to managing respiratory conditions like asthma.
- Motion sensors (for smart homes) help to monitor activity levels and detect falls. They are particularly beneficial for elderly or disabled individuals.
These medical IoT sensors work together as part of a larger system. They send information to the cloud, where it undergoes an ETL process (Extract, Transform, Load) to prepare it for analysis. This data can then be used for various purposes, such as:
- Monitoring health trends
- Providing early warnings of potential problems
- Sending emergency notifications
By collecting and analyzing this data, medical professionals and individuals can gain valuable insights into patient health and well-being.
But how exactly do such valuable sensors work?
MIoT system orchestration: What to consider
We’ve explored how medical IoT device monitoring collect valuable health data. But how does it all come together? Custom IoT consulting services help healthcare organizations design and implement robust medical IoT architectures. Here’s a simplified look at the system architecture of such digital health solutions.
4 layers of communication that enable medical IoT devices to share necessary medical data:
1. Sensor layer includes sensors we’ve discussed earlier. Here the data accumulated from IoT medical devices is sent to the network layer after initial processing.
2. Network layer transmits data (i.e. vital signs) from the sensors to the cloud. It uses various wireless technologies like Wi-Fi, 4G/5G, LoRa, BLE, and ZigBee. The most common communication protocol within this layer is MQTT broker protocol.
3. Cloud layer: Once data reaches the cloud, it undergoes an ETL process to prepare it for analysis. Here’s what happens next:
- Analysis and alerts. The system analyzes the data and may trigger notifications based on predefined rules. These notifications could be sent to doctors, ambulances, or other relevant parties.
- Data storage. The data is stored in a cloud database for future analysis or reference.
- Advanced processing. Stored data can be used for batch analysis by business intelligence tools and machine learning algorithms (use cases for machine learning).
4. Application and presentation layer, where the processed information is presented in a user-friendly format on applications or dashboards, allowing for easy access and better health management.
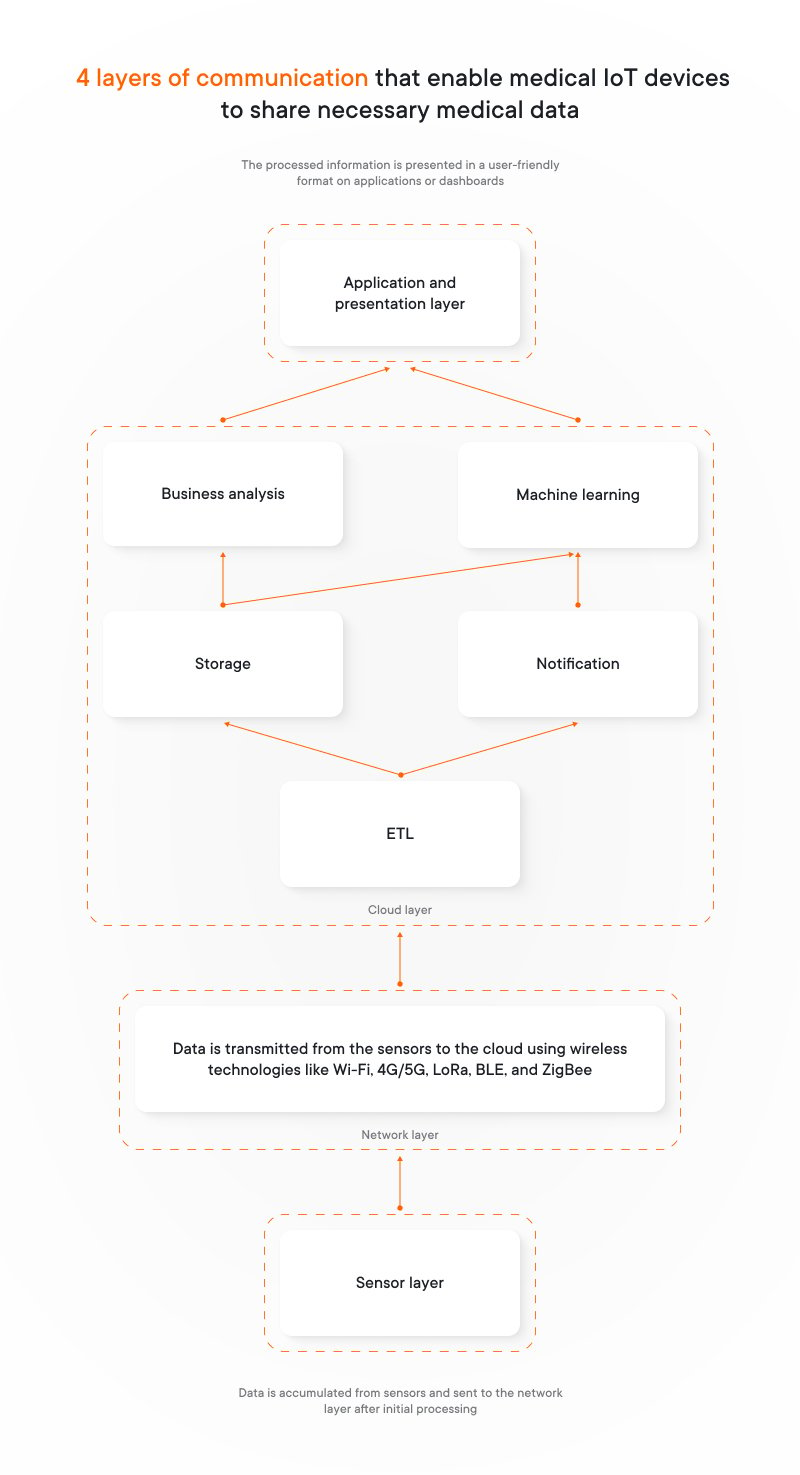
This layered architecture ensures efficient data collection, communication, analysis, and presentation, providing valuable insights into health and well-being.
Although the architecture seems simple and understandable, it’s not easy to work with IoT-powered healthcare systems due to the challenging process of sensor integration. Let’s get deeper into it.
Challenges and considerations in sensor integration: How IoT healthcare devices change the game
Integrating sensors into MIoT devices comes with a unique set of challenges that must be carefully addressed to ensure accurate data and safe operation.
Challenges of sensor integration
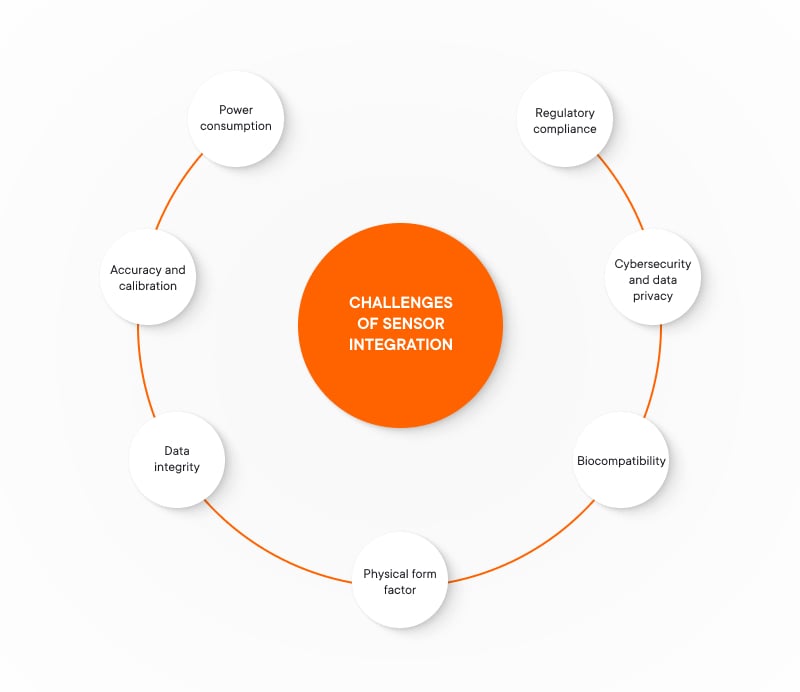
- Power consumption. Many sensors, especially in wearable devices, rely on batteries. Balancing power efficiency with continuous healthcare IoT device monitoring is crucial to avoid frequent recharging.
- Accuracy and calibration. Medical data needs to be highly reliable. Sensors must be meticulously calibrated and regularly validated to ensure accurate readings, which are critical for correct diagnosis and treatment.
- Data integrity. Distributed systems raise concerns about data integrity due to potential sensor drift or signal interference. IoT healthcare devices need robust error-checking and validation mechanisms to guarantee data accuracy during transmission.
- Physical form factor. Sensors in connected medical devices, particularly wearable devices, need to consider size and comfort for the user. Sensors should be miniaturized and seamlessly integrated to avoid hindering patient movement.
- Biocompatibility. IoT devices in healthcare with direct patient contact must use biocompatible materials to prevent allergic reactions or tissue damage. Sensors should be rigorously tested before deployment in a medical setting.
- Cybersecurity and patient data privacy. MIoT devices collect sensitive health data. Secure transmission protocols, encryption, and strong user authentication are essential to protect patient privacy and prevent unauthorized access to health information. Collaborating with IoT application security services ensures that medical IoT devices implement advanced security measures, from secure data transfer to compliance with healthcare regulations, safeguarding both patient information and device integrity.
- Regulatory compliance. Healthcare IoT devices are heavily regulated. MIoT devices must adhere to stringent standards set by agencies like the FDA to ensure safety and effectiveness.
So how to address them to create a product that will be highly praised by healthcare providers and patients, let alone approved by governmental bodies?
Considerations for successful integration
Here are some key aspects to consider on how to integrate IoT in medical devices:
Rule #1: Early collaboration
From the initial design stages, engineers, medical professionals, and data scientists should collaborate. This ensures IoT devices for healthcare sector are suitable for the medical context and address real clinical needs. Engaging IoT product development services at an early stage helps streamline the design and technical implementation of medical IoT devices, ensuring they meet both clinical requirements and patient expectations.
Rule #2: Robust testing and validation
Rigorous testing and validation processes are essential. These ensure sensor accuracy, reliability, and overall device safety under various conditions.
Rule #3: Data handling and analytics
MIoT systems must incorporate data storage, security protocols, and analytic tools. This transforms raw sensor data into actionable insights that can be used to improve patient care and patient outcomes.
Following these core principles lays the groundwork for successful medical IoT development services. But to translate these principles into a real-world product, we need to delve deeper. Here’s where the crucial stages of prototyping, compliance, and quality assurance come into play. Let’s talk about each of them.
Grab your free checklist! Learn the best healthcare IoT testing practices from our experts
Get the checklistThe role of medical IoT devices prototyping in future-proving innovations
Prototyping plays a critical role in the development of IoT devices used in healthcare, serving as a bridge between conceptualization and full-scale production. This iterative process allows for:
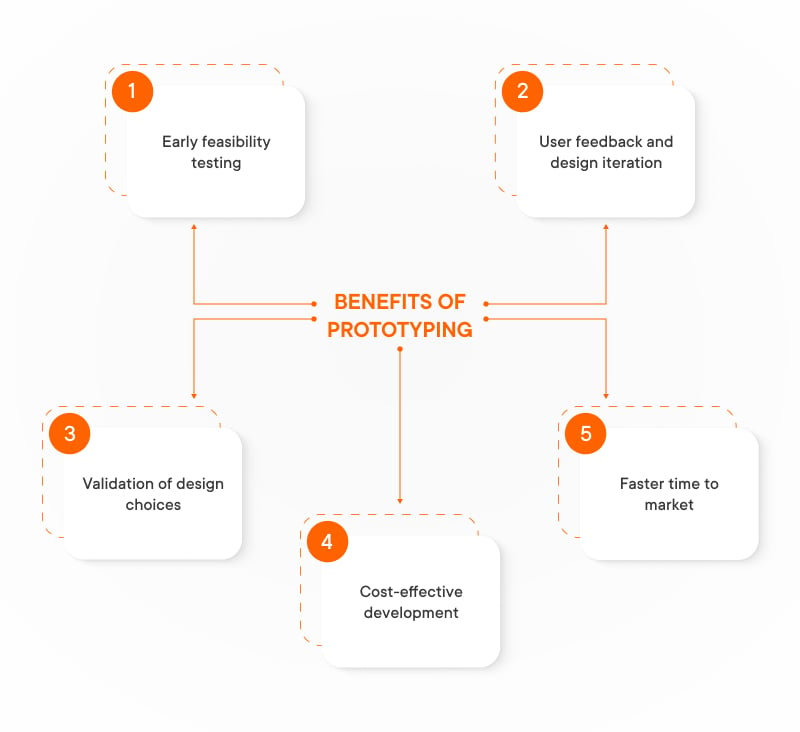
- Early feasibility testing. Prototypes enable early testing of sensor integration, data collection capabilities, and overall device functionality. This helps identify potential issues early on, saving time and resources in the medical device IoT development process.
- User feedback and design iteration. Prototypes can be used to gather feedback from potential users, such as patients, healthcare professionals, and caregivers. This feedback helps refine the design, ensuring IoT medical devices meet user needs and are comfortable and intuitive to use.
- Validation of design choices. Prototypes allow for testing various design choices, including sensor placement, battery life, and user interface design, before committing to large-scale production. This ensures the final product is optimized for functionality and user experience.
- Cost-effective development. Prototyping is significantly cheaper than full-scale production. This allows for exploring multiple design iterations without incurring significant financial risks, maximizing the potential for success in the final product.
- Faster time to market. By identifying and addressing challenges early through prototyping, companies can bring innovative IoT medical devices to market faster, allowing patients to benefit from the latest advancements in healthcare technology sooner.
While prototyping helps refine the technical aspects of an MIoT device, ensuring its real-world effectiveness and safety requires more. This is why compliance and quality assurance (QA) are the next things you have to take care of. They bridge the gap between a promising prototype and a reliable medical device that can be confidently used in the healthcare industry.
Let’s explore why compliance and QA are essential for successful MIoT development in the medical field.
Ensuring safety and trust: Compliance and Quality Assurance in medical IoT
IoT medical devices holds immense potential for changing healthcare. Wearables monitoring vital signs, smart inhalers tracking medication use, or ingestible sensors diagnosing illnesses – these are just a few possibilities. However, with great potential comes great responsibility. Unlike fitness trackers, medical IoT device monitoring requires dealing with sensitive health data and directly impact patient well-being. This is where compliance and quality assurance (QA) become critical cornerstones of responsible development.
Compliance: Why the rules matter
IoT medical devices are subject to strict regulations set by agencies like the FDA (US Food and Drug Administration) or CE marking (Europe). Besides, there are also such regulations as IEC 62304, HIPAA and others that a healthcare business must follow depending on the country they operate in. Yalantis constantly improves our knowledge of such regulations and assists our clients in obtaining them and making their systems compliant. For example, we helped one of our clients to obtain ONC Health IT Certification.
Healthcare regulations ensure the connected medical devices are safe, effective, and meet specific performance standards. Compliance is not just about ticking boxes — it’s about prioritizing patient safety. This extends beyond your future product; it also concerns the security practices your software development partner utilizes. To ensure our partners’ peace of mind, Yalantis follows all necessary internal security practices and undergoes official audit and certification programs, as we did with ISO/IEC 27001, ISO 9001, and ISO 27701.
Here are more reasons why compliance matters:
- Safety first. Regulations address potential risks associated with the device, minimizing the chance of malfunction or harm to patients.
- Data security. Compliance ensures patient data privacy and security, preventing unauthorized access or breaches.
- Accurate information. Regulations guarantee that the data collected by the device is reliable and can be trusted by healthcare professionals.
Compliance mistakes cost more than you think
Cut rework and service costs before they happen with our free ISO 13485 + FDA compliance guide.
Quality assurance: Building confidence
Imagine a blood pressure monitor giving inaccurate readings – the consequences could be dire. Quality assurance aims to minimize such risks. QA involves testing the device throughout its development lifecycle, ensuring it functions as intended and meets all design specifications.
Here’s how QA strengthens medical IoT development:
- Early detection. QA helps identify and fix potential issues early on, saving time and resources in the long run.
- Reliable performance. Thorough testing ensures the device performs consistently and reliably under various conditions.
- Improved user experience. QA contributes to a user-friendly device that is easy to use and provides accurate information.
By prioritizing both compliance and QA, developers can build IoT for medical devices that are not only technologically advanced but also safe, reliable, and trustworthy.
Wrapping up
Now, we can confidently assume that the future of the healthcare industry is definitely with medical IoT. However, this vision requires the integration of various technological stacks and domain areas.
The continued development of diverse sensor integration, robust prototyping practices, and a focus on patient needs will be crucial in seeing what MIOT full potential is. As this field continues to evolve, we can expect even more innovative IoT medical devices and applications to emerge (IoT application development), shaping a future where healthcare is personalized, preventative, and accessible for all.
Need a reliable tech partner for IoT medical device development?
FAQ
How IoT devices contribute to improved healthcare delivery?
IoT medical devices transform healthcare by enabling continuous patient monitoring and real-time data sharing with clinicians. Instead of relying solely on periodic checkups, doctors gain ongoing insights into vital signs such as heart rate, glucose levels, and blood pressure. This constant flow of data allows for early detection of complications, faster response times, and more personalized treatment plans. Patients also benefit from greater independence, as they can manage chronic conditions at home while staying connected to healthcare providers. Overall, IoT in medical devices bridges gaps between patients and clinicians, improving outcomes and reducing hospital readmissions.
What types of medical IoT devices do you develop?
We work on a wide range of medical IoT devices tailored to different clinical needs. These include wearable devices for continuous health tracking, such as ECG monitors, glucose sensors, and blood pressure monitors, as well as environmental sensors that ensure safe patient conditions. We also develop platforms that integrate multiple devices into unified healthcare ecosystems, enabling doctors to monitor patient progress remotely and in real time. Each solution is designed to balance usability, accuracy, and regulatory standards, ensuring the highest level of trust from both patients and clinicians. Our expertise covers connected medical devices in the Internet of Things.
How do you ensure compliance in Medical IoT projects?
Compliance is at the core of every medical wearable IoT development project we handle. We design systems to meet strict regulatory requirements, including FDA, CE, IEC 62304, and HIPAA, depending on the market and device type. Our teams follow industry best practices for risk management, clinical data validation, and medical device IoT security to guarantee both patient safety and data protection. Regular audits, validation testing, and quality assurance processes ensure that the final product aligns with regulatory expectations. By embedding compliance into every stage, no matter if it’s prototyping or deployment, we help clients bring safe and certified IoT medical devices to market.