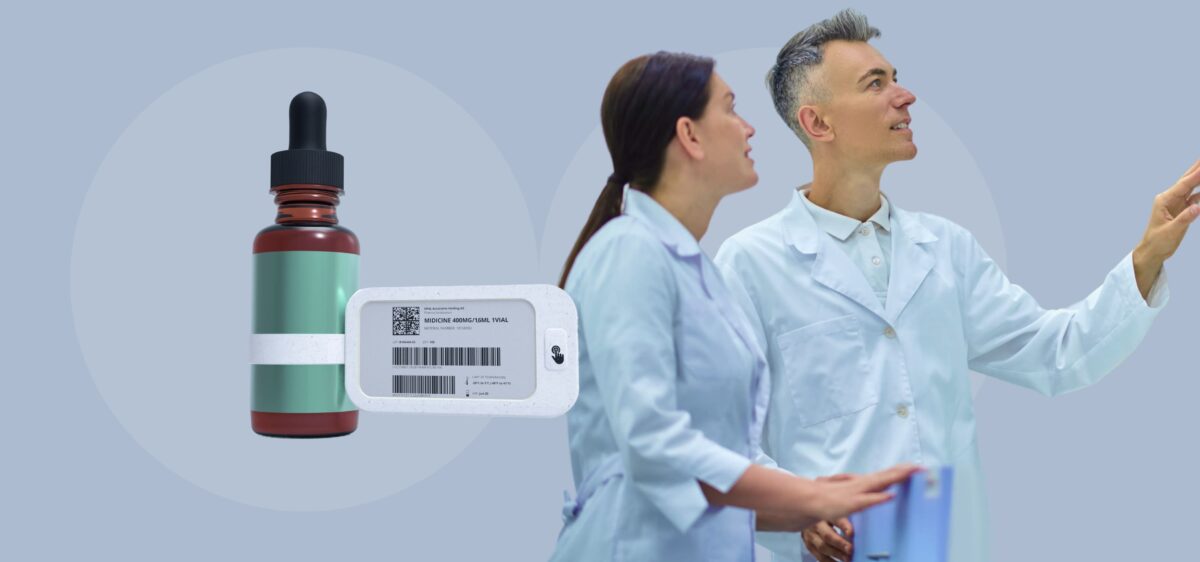
Replace manual paper relabeling with a digital solution
Pharma Company Cuts Relabeling Time from 2 Months to 1 Day with Digital Display Labels
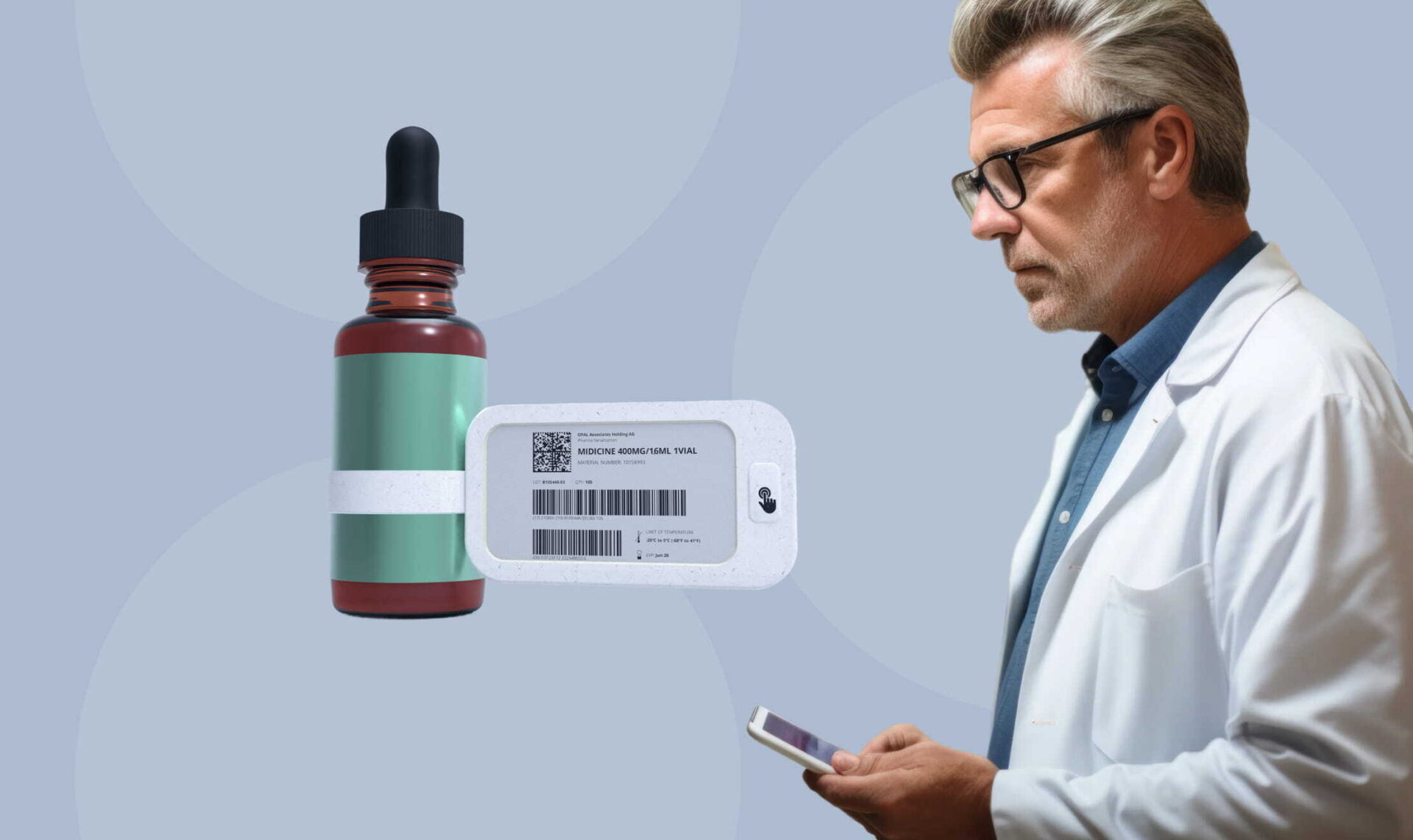
Yalantis developed custom digital display labels for clinical trials, helping a pharmaceutical company transition from manual paper labeling to a fully digital system.
Relabeling time
Saved in shipping
Reduced IMP waste
“Our client spent up to 8 weeks on each relabeling cycle, and drug expiry dates could change multiple times. So they came to us for custom digital display labels.”
– Mykhailo Maidan, CTO at Yalantis
Need a custom IoT device? We can build it full-cycle.
Be our next success story. Share details about your project and book a call with us to discuss your goals.
From medical devices to industrial automation — we deliver complete enterprise solutions with regulatory compliance built-in. Everything under one roof.
Our offices
Poland
123 al. Jerozolimskie, Warsaw, 00-001
Ukraine
5 Dmytra Yavornytskoho Avenue, Dnipro, 49005
Cyprus
8 Athinon Street, Larnaca, 6023
Estonia
12 Parda, Tallinn, 10151
FAQ
-
What compliance and validation coverage did your team deliver?
The full solution (hardware, firmware, app, and integration) was validated under GxP/data-integrity standards, aligned with FDA 21 CFR Part 11 and EU GMP Annex 11, and it passed internal audits and verification reviews as well as formal compliance audits.
-
How does the digital labeling system integrate with clinical systems?
It plugs into your CTMS/inventory stack and pushes every on-site update to the central DB in real time, giving QA/Supply full visibility.
-
How are security and auditability handled during label updates?
When a provisioned device scans a label, the firmware authenticates the connection, writes updated data, verifies integrity with checksums, and refreshes the e-ink display within seconds. The mobile app logs every action for traceability, and updates are synchronized to the central system for oversight.
-
How did you prove the hardware’s reliability in real conditions?
Our team selected compliant components (battery, chip, shielding) designed for sharp temperature swings, chose e-ink for readability/low power, and built functional prototypes that were later tested in real-world conditions.
-
Can the solution handle scale and multilingual content?
Clinical staff can update hundreds of kits in minutes on-site, and enabling real-time, multilingual label updates at clinical sites was within the project scope.
-
What did you tackle to stabilize the system and de-risk operations?
Yalantis designed, built, integrated, and validated the system in 5 months, cutting relabeling from ~8 weeks to ~24 hours, saving $750K in shipping and reducing IMP waste by 90% in the referenced trial.
Let’s Start from call scheduling
- Schedule a call
- We collect your requirements
- We offer a solution
- We succeed together!
Thank you for contacting us.
Meanwhile, you can explore our hottest case studies and read
client feedback on Clutch.
We are open for partnerships too
Check out our refferal program. Find out all benefits.