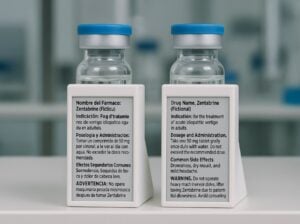
Paper labels are failing pharmaceutical companies at the worst possible moments.
A vial sits in a -80°C freezer, its label slowly peeling away from condensation damage.
A radioactive cancer drug expires before its label can even be printed.
A clinical trial grinds to a halt because extending a product’s shelf life requires physically recalling and relabeling thousands of units, a process that takes weeks the product does not have.
These are not edge cases. These are daily operational realities in pharmaceutical supply chains, particularly for biologics that require extreme cold storage and therapies with shelf lives measured in hours rather than months. The root problem is obvious: paper labels are static objects in a dynamic system. They cannot adapt to new information, they deteriorate under environmental stress, and updating them requires manual processes that consume more time than many modern therapies can afford.
Digital display labels offer a fundamental shift in how pharmaceutical products carry information. Instead of ink printed on paper and attached with adhesive, these electronic screens display regulatory content that can be updated remotely through wireless signals. The technology has been used in retail and logistics for decades, but its application to pharmaceutical cold chains (especially for clinical trials and temperature-sensitive biologics) introduces capabilities that paper labels simply cannot match.
The questions facing pharmaceutical operations leaders are practical: Can these displays survive -80°C storage? How do you update a label on a product with a 72-hour shelf life? What happens to cold chain procedures when labels become dynamic instead of static? The answers determine whether this technology can move from prototype to standard practice across an industry where regulatory compliance and patient safety leave no room for failure.
What are the unique labeling challenges for cryogenic and ultra-low temperature products?
Extreme cold does terrible things to paper labels. Anyone who has pulled something from a deep freezer knows this intuitively, frost builds up, moisture seeps in, and stickers start peeling off.
In pharmaceutical cold storage, where temperatures drop to -80°C or lower, these problems become critical. The adhesive that holds labels onto vials loses its grip. Ink becomes brittle and chips away like old paint. When workers move products between freezers and refrigerators, condensation forms and washes away whatever text remains readable. A label that was perfectly legible in the morning might be illegible by afternoon.
This creates a cascade of problems. If you cannot read the lot number, you cannot trace where the product came from. If the expiry date has faded, you cannot verify whether the product is still usable. And if you cannot confirm what is in the vial, you certainly cannot give it to a patient.
The space issue compounds these problems. Tiny vials do not have much room for labels to begin with. Add the insulation and specialized containers needed for cryogenic storage, and there is even less surface area to work with. When regulations require the label to include information in multiple languages, something has to give, usually readability. The font shrinks so small that even people with perfect vision struggle to read it.
Relabeling is a painfully slow solution. You have to pull the products out of frozen storage, transport them to a qualified packaging facility, manually apply new labels, verify the work, and return everything to the freezer. Each step takes time. Each step risks temperature excursions that could damage the product. For cell therapies that must stay at -80°C, a single relabeling cycle can take weeks and generate multiple deviation reports if anything goes wrong.
Digital display labels sidestep these issues entirely. Because the information appears on an electronic screen rather than printed paper, there is no ink to fade and no adhesive to fail. The display keeps working regardless of temperature, and you can update the information remotely without ever touching the product.
How to manage expiry date updates for products with a shelf life of only 72 hours?
Some drugs decay so fast that traditional labeling cannot keep up.
Take radioligand therapies, molecules used to treat certain cancers. These compounds are radioactive by design, which makes them effective but also means they decay rapidly. Many have shelf lives of just 72 hours. The clock starts ticking the moment they come out of manufacturing, and it does not stop.
Here is the problem: designing, printing, and applying a paper label takes longer than 72 hours. Label design requires 3 to 5 days for approval. Printing takes another 1 to 2 days. By the time the label is ready, the drug has already expired. The product becomes waste before it even gets packaged.
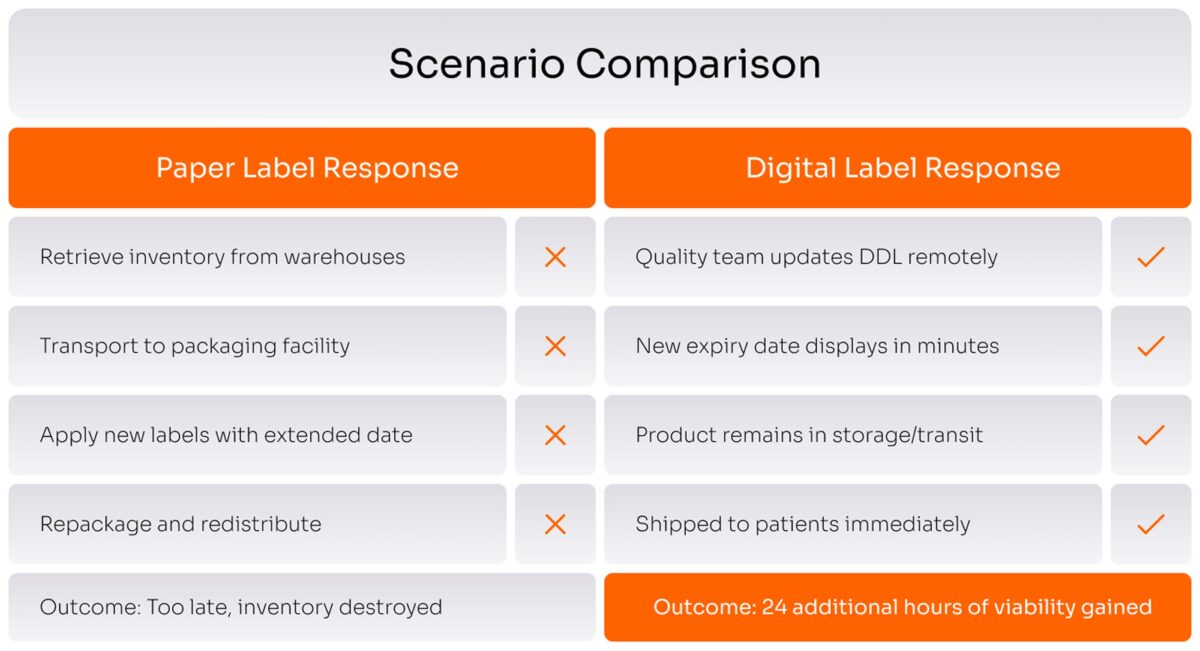
Now add another complication. Drug companies routinely conduct stability testing to see if products can safely last longer than initially approved. When new data shows a 72-hour product can actually last 96 hours, that is valuable, it gives you 24 more hours to get the drug to patients who need it.
But with paper labels, capturing that value is nearly impossible. You would need to physically retrieve products from multiple warehouses, bring them to a packaging facility, apply new labels with the extended date, and ship them back out. The logistics are absurd. Most companies just throw the inventory away rather than attempt this dance.
Digital labels change the math completely. When new stability data extends the shelf life, a technician in the quality department can update the label remotely through the computer system. The new expiry date appears on the display within minutes. The product stays exactly where it is: in the freezer, on the truck, or at the hospital. No recall, no relabeling, no wasted time.
This matters most when supply is tight. During shortages, extending shelf life on existing inventory by even a single day can mean some patients get treatment instead of waiting. For drugs treating aggressive cancers or life-threatening conditions, those 24 hours make a real difference.
Can electronic labels operate reliably in frozen conditions (e.g., -80°C)?
The short answer: sort of, but it works better than you might think.
Electronic displays use tiny particles suspended in fluid to create text on a screen. When temperatures drop below about -20°C, that fluid becomes too viscous and the particles stop moving. You cannot update the display while it is frozen.
But here is the clever part: e-paper displays do not need power to maintain an image once it appears. Write something to the screen, and it stays there indefinitely, even at -80°C, even with no battery. The display is essentially frozen in place, quite literally, but it remains readable.
This works because the practical workflow allows for updates at strategic points. Labels get updated before products go into deep freeze, or during routine handling when products temporarily come up to room temperature. For drugs that require continuous ultra-cold storage, the initial label contains all the essential information needed for the product’s entire life cycle.
Consider how cold chain logistics actually operate. Products do not stay frozen every single moment. They come to room temperature during depot inspections, inventory checks, and preparation for dispensing. These moments provide windows to update labels when needed.
Engineers are working on displays that function at lower temperatures, testing new materials and alternative technologies. Still the current approach already works for most real-world situations because it aligns with how people actually handle frozen products.vDigital labels do not need to update while frozen. They just need to retain information while frozen and accept updates during normal handling. Current technology already does this reliably.
How can labeling technology integrate with temperature monitoring systems?
Right now, temperature monitoring and product labeling operate as separate systems, which creates gaps that compromise product safety.
Walk into any pharmaceutical warehouse and you will see temperature data loggers everywhere, small devices that record whether freezers and refrigerators stay within acceptable ranges. These loggers do their job, but they only tell you about the environment, not about specific products. If a freezer malfunctions at 3 a.m., you know the temperature spiked, but figuring out which products were affected requires cross-referencing shipping records, inventory logs, and batch numbers. It is manual detective work.
Digital labels can combine both functions on the product itself. The same hardware that displays label information can include a temperature sensor. Every product becomes its own monitor, recording its complete temperature history from factory to patient. No more wondering whether a specific vial got too warm during shipping, the data travels with the vial.
The system architecture builds on infrastructure that already exists. The same wireless technology used to update label content can transmit temperature readings back to the central database. When readings exceed safe limits, the system automatically alerts the quality team and updates the product’s label to flag the issue. Hospital pharmacists see the temperature excursion status immediately without downloading separate logger files or making phone calls.
This integration enables smarter decision-making across the supply chain. When temperature data from hundreds of shipments reveals that certain routes or carriers consistently have problems, your team can adjust before products get damaged. Real-time monitoring catches issues while they are still fixable rather than discovering them weeks later during routine audits.
The cost savings are straightforward. Manual temperature documentation consumes hours of labor per week. Investigating temperature excursions, figuring out what happened, which products were affected, whether they are still safe… these all generate mountains of paperwork. Automatic detection and documentation cuts this work dramatically while improving product safety and regulatory compliance.
Go from reacting to temperature excursions to proactively optimizing your supply chain.
Let’s use integrated data to identify and fix issues before they happen.
What is the risk of paper label failure (e.g., ink fading, falling off) in cold chain logistics?
Paper label failure in cold environments is not an occasional inconvenience. It is a recurring source of major operational problems.
The damage happens through multiple mechanisms. Water-based inks crystallize when frozen, causing text to separate and fade. Toner used in laser printing becomes brittle in cold. When condensation forms during temperature changes (which happens constantly in cold chain logistics) ink bleeds or washes away entirely.
When critical information becomes unreadable, you face a binary choice: investigate extensively or destroy the product. If the lot number is gone, traceability is lost and regulations prohibit using the product. If the expiry date cannot be read, you cannot verify the product is still good. If dosing instructions blur, giving it to a patient would be unsafe. Each scenario triggers formal deviation reports, investigations to determine root cause, and corrective action plans. The administrative burden often costs more than the product itself, so companies default to destruction.
Adhesive failure poses parallel risks. Below -30°C, most adhesives lose their stickiness. Labels peel off curved surfaces like vials during routine handling. Once a label detaches completely, the product becomes unidentifiable and unusable. This is particularly devastating for autologous cell therapies where each unit is manufactured specifically for one patient. A lost label means a lost treatment with no replacement possible.
The financial damage accumulates at every stage. Manufacturing sites add weeks to release schedules for enhanced label testing. Warehouses hold shipments for incoming inspections to verify label integrity. Hospitals report label issues as deviations, triggering investigations by the sponsoring company. A single label failure can cascade into months of documentation and tens of thousands of dollars in investigation costs.
Digital displays eliminate these physical failure modes. Electronic screens do not use ink that can fade or adhesive that can fail. The information lives in computer memory, unaffected by temperature, moisture, or handling stress. What you see on day one is what you will see on day 365, regardless of how many times the product has been frozen and thawed.
How can Digital Labels boost the release process for time-critical therapies like radioligands?
Radioactive drugs represent an extreme case where normal pharmaceutical logistics break down completely. The product decays on a timeline measured in hours while regulatory processes are measured in days.
Traditional release follows a strict sequence. Quality control testing takes 12 to 24 hours. Label design and approval take 3 to 5 business days. Printing requires 1 to 2 days including setup and verification. Physical labels must be applied during packaging. This workflow assumes you finalize labels before manufacturing begins. A reasonable assumption for drugs with multi-year shelf lives, but absurd for radioligands.
Consider Lutetium-177 therapies with a 6-7 day half-life. Manufacturing schedules work backward from patient appointment times. You calculate when the drug needs to reach the hospital, add transit time, add quality control time, add manufacturing time, and start production accordingly. There is no margin for delays.
Here is where paper labels become a bottleneck. By the time you have stability data to set an accurate expiry date, the product is already packaged and heading out the door. Any changes require recalling products, relabeling in a qualified facility, and reshipping, consuming more time than the product will remain viable.
Digital labels eliminate this bottleneck by separating physical packaging from information finalization. Products can be manufactured, filled, and sealed with blank electronic displays attached. As quality control confirms specifications, the system calculates the decay-adjusted expiry date and loads the complete label content to the display via wireless signal. This happens minutes before shipment, not days before manufacturing.
This capability unlocks pooling strategies impossible with paper. Multiple clinical trial protocols can share common unlabeled inventory. When a specific patient needs treatment, the system assigns product from the pool and applies the protocol-specific label on demand. Inventory becomes flexible while waste from protocol-specific overproduction decreases.
Expiry extensions gain particular value here. If stability data supports extending a 72-hour product to 84 hours, the digital label updates while the product is already in transit or sitting in the hospital pharmacy. Paper labels would require recalling the product for relabeling, consuming more time than the extension provides. Thus, making the exercise pointless.
The operational gain translates directly to patient access. Reducing release time from weeks to days means more patients receive treatment within optimal therapeutic windows. For cancers where radioligands provide last-resort therapy, this acceleration is clinically meaningful.
Unlock inventory pooling and on-demand labeling for your radiopharmaceuticals.
Let’s build the hyper-flexible supply chain these critical therapies demand.
Does the use of Digital Displays require changes to existing cold chain SOPs?
Implementing digital labels does require updating standard operating procedures, but the changes are less disruptive than you might expect.
Current procedures specify how workers should inspect paper labels: check print quality, verify adhesive attachment, confirm information accuracy. These inspection steps need to adapt for electronic displays that show information differently than ink on paper. New criteria include verifying the display is functional, checking battery status where applicable, and confirming what appears on screen matches electronic records in the system.
The biggest procedural shift involves update authorization. Paper labels are permanent, once applied, they cannot change without physically relabeling the product. Digital labels are dynamic. Procedures must define who has authority to update labels, under what circumstances updates are permitted, and what verification happens before and after changes. This includes change control procedures, approval workflows, and audit trail requirements to maintain regulatory compliance.
Facility qualification changes fundamentally. Paper label operations require dedicated packaging areas where trained personnel apply labels under controlled conditions. Digital label updates can happen anywhere with wireless connectivity: warehouse storage areas, distribution centers, even hospital pharmacies. Procedures must establish that these locations meet appropriate environmental and security standards for electronic label operations.
Documentation shifts from physical to digital. When paper labels fail, investigators take photos of damaged labels, attach label samples to batch records, and include reproductions in investigation reports. Digital label documentation captures screenshots, system logs, and electronic audit trails.
However, core cold chain requirements remain unchanged. Temperature monitoring, storage conditions, handling procedures, and stability protocols stay identical. Digital labels do not alter the physical properties of products or their storage requirements. Procedures for receiving, storing, managing inventory, and dispensing require modification only in the label verification and documentation sections, not wholesale rewrites.
Most organizations use a phased approach. Initial implementations operate under pilot protocols that keep existing procedures in place while documenting digital label-specific steps separately. Lessons from pilot phases inform final procedure revisions. This reduces risk and allows validation of new approaches before full implementation.
The validation documentation provided by digital label system vendors includes evidence that the technology maintains product quality and data integrity. This package supports regulatory inspections and demonstrates that cold chain integrity is maintained or improved with the new system.
Conclusion
The case for digital display labels in pharmaceutical cold chains comes down to a simple reality: paper cannot keep up with modern drug development. When therapies decay in hours, when storage temperatures approach absolute zero, and when clinical trials require supply chain agility that traditional labeling cannot provide, the limitations of ink and adhesive become operational barriers rather than minor inconveniences.
The technology is not without challenges. Electronic displays cost more upfront than paper. Procedures need updating to accommodate dynamic rather than static labels. Organizations must invest in system integration, validation, and training. Regulatory agencies are still developing guidance for this approach, and early adopters face the burden of establishing precedent. These are real obstacles that deserve serious consideration.
But the alternative is maintaining a status quo that generates preventable waste, creates compliance risk, and limits patient access to time-critical therapies. When a label failure means destroying a personalized cell therapy that cannot be replaced, or when manual relabeling processes consume more time than a radioactive drug remains viable, the cost of inaction becomes clear. Digital labels address problems that paper labels fundamentally cannot solve, and the industries that adopt this technology first will gain operational advantages that competitors cannot easily replicate.