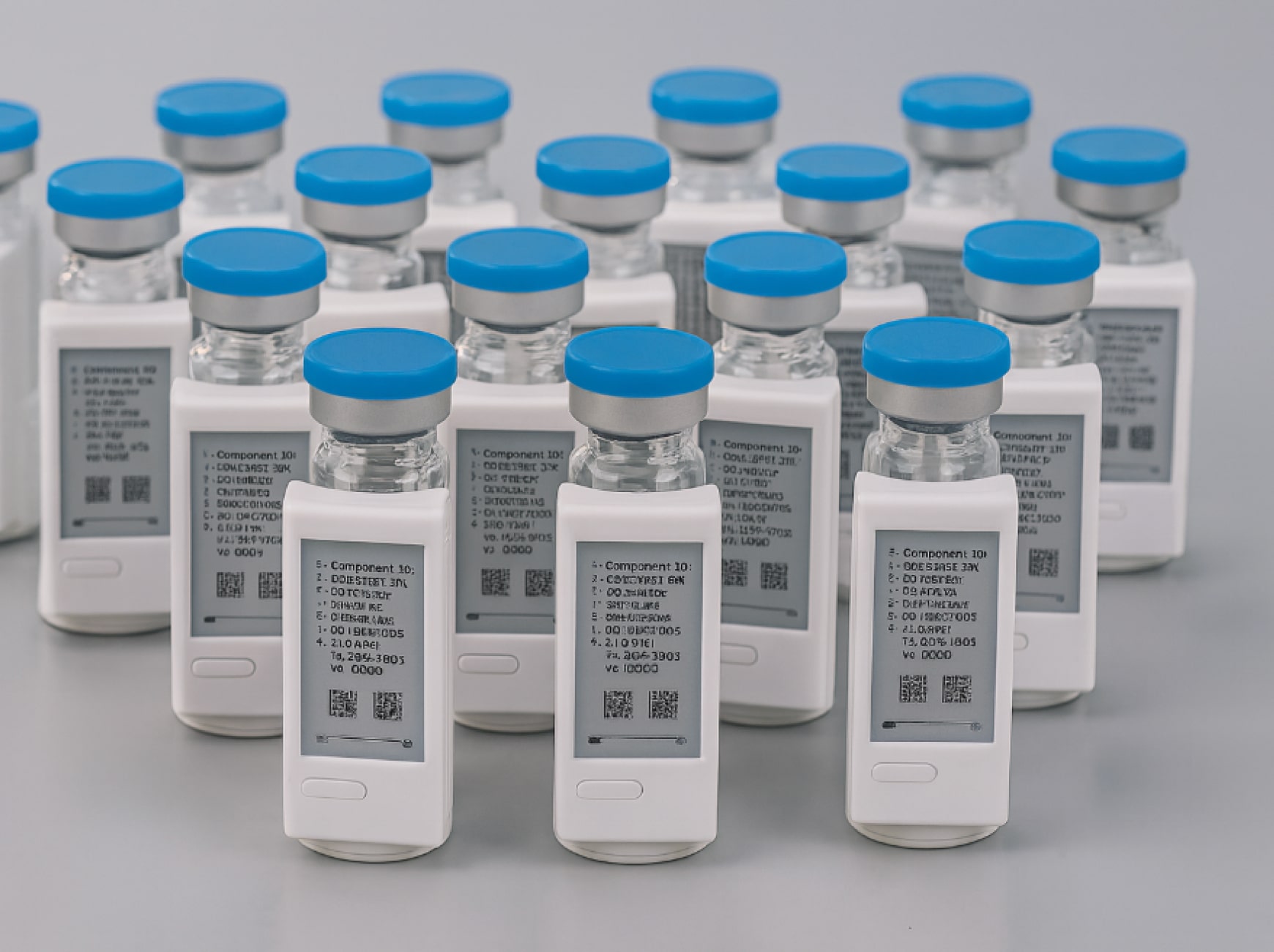
Paper labels on clinical trial medications create a cascade of problems that pharmaceutical companies have tolerated for decades. When stability testing extends a drug’s expiration date during a long-running trial, every vial, bottle, and blister pack must be physically recalled, relabeled in qualified facilities, and redistributed. This process consumes weeks, generates significant waste, and can leave patients without access to investigational treatments they depend on.
Manual printing and application processes generate labeling-related deviations that account for a significant portion of quality events at major pharmaceutical companies. Wrong templates get selected. Incorrect data gets entered. Labels are applied to the wrong products or positioned improperly. Each mistake requires investigation, corrective action, and often product destruction.
Digital Display Labeling addresses these fundamental problems by replacing static paper with electronic displays that can be updated remotely through validated, system-controlled processes. The question facing pharmaceutical executives is no longer whether this technology works but rather how quickly their organizations can implement it to reduce costs, improve supply chain agility, and accelerate patient access to investigational therapies.
What is Pharmaceutical Digital Display Labeling?
Digital Display Labeling replaces traditional paper labels on investigational medicinal products with electronic displays, typically using e-paper technology similar to e-readers. These digital labels attach directly to clinical trial packaging such as vials, bottles, blister packs, and syringes, displaying all required regulatory information as human-readable text.
The core distinction between digital labels and paper labels lies in their ability to be updated remotely. Where a paper label remains static from the moment it prints, a digital label can receive content updates through wireless communication technology, specifically NFC. This means that critical information like expiration dates, storage instructions, or dosage details can change without physically handling the product.
Each digital label contains a unique identifier that connects to enterprise resource planning systems, creating a traceable chain of custody for every content change. Current digital labels can display multiple pages of information. Users navigate through content using physical buttons on the device, eliminating the need for smartphones or external readers. This independence from secondary technology addresses a key regulatory concern about universal accessibility.
The display itself uses no power when showing static content, only drawing from a small battery during updates. Current designs aim toward reusable labels that can be removed, sterilized, and reapplied to new products, though this remains under development for broader implementation.
When and How Digital Display Labeling is Being Used in Pharmaceutical Clinical Trials
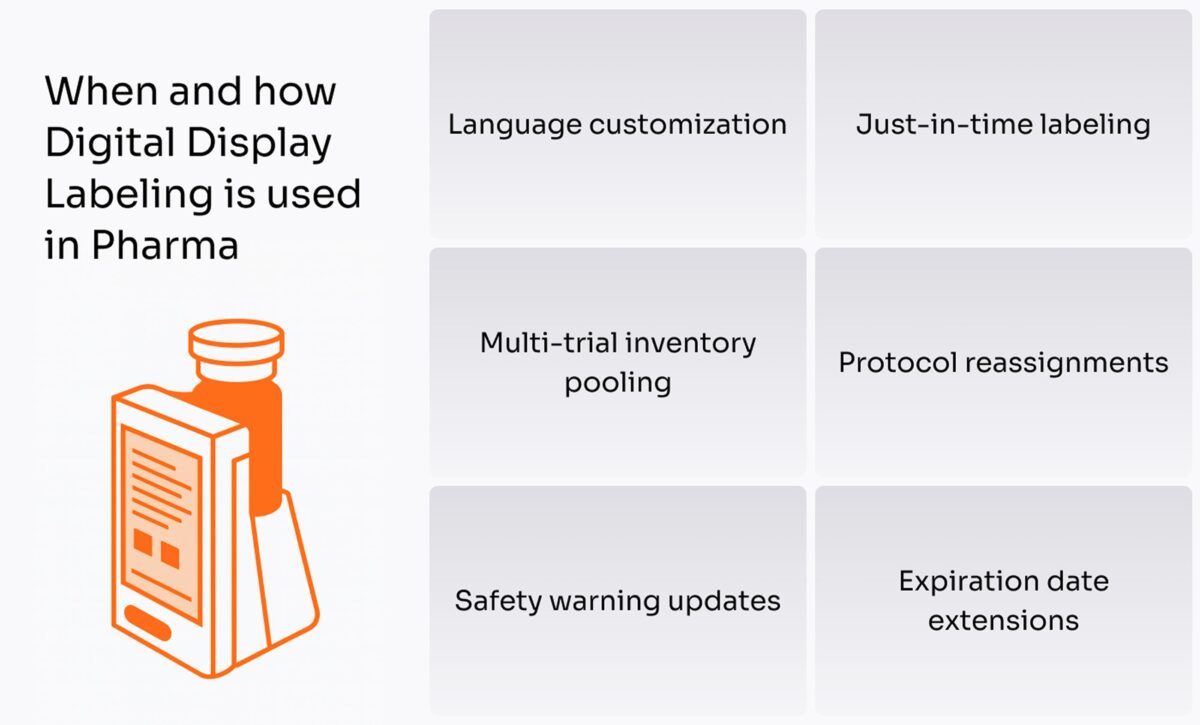
Digital Display Labeling currently operates in specific, high-value scenarios where traditional paper labels create the most friction. The primary use case involves expiration date extensions during long-term clinical trials.
Consider a Phase III trial testing a chronic disease treatment over 24 months. Products manufactured at the trial’s start may have initial expiration dates falling within the trial period. When stability testing proves the drug remains effective longer, extending the expiration date requires relabeling every unit in the supply chain. With paper labels, this means recalling products from distribution centers or clinical sites, applying new labels in qualified facilities, and redistributing them. The process typically takes four to six weeks and introduces risk at every manual step.
Digital labels eliminate this entire workflow. A validated change to the expiration date in the enterprise system triggers wireless updates to affected labels within days, sometimes hours. The product never leaves its storage location. Clinical sites receive notification of the update, and patients continue treatment without interruption.
Language customization is an another active use case. Global trials enroll patients across dozens of countries, each requiring labels in local languages. Traditional approaches produce country-specific packaging or use multilingual booklet labels with small, difficult-to-read text. Digital labels store content in multiple languages, allowing clinical site staff to select the appropriate language for each patient. This supports both regulatory compliance and patient safety by ensuring instructions are clearly understood.
Several pharmaceutical companies have conducted pilots testing digital labels in real clinical settings in recent years. These pilots focus on safety monitoring trials and studies where supply flexibility provides the greatest value. One documented pilot successfully updated expiration dates on investigational products at a clinical site, demonstrating feasibility within current regulatory frameworks.
The technology also enables just-in-time labeling strategies. Rather than applying final labels during manufacturing, companies can place digital labels showing only basic product identification. Complete labeling occurs at distribution centers or clinical sites just before dispensing, incorporating the most current information about the specific trial, patient, or protocol. Pooling inventory across multiple clinical trials becomes more practical with digital labels. A single batch of investigational products can be assigned to different protocols by updating the digital label rather than maintaining separate, protocol-specific inventory.
Eliminate the friction with instant, wireless updates.
Tired of the 4-6 week process of recalling and relabeling for expiration date extensions?
What are the Technical Requirements to Implement Digital Display Labeling in Pharma
Implementing digital display labeling requires careful integration of hardware, software, and operational processes that meet pharmaceutical manufacturing standards.
The physical digital label must withstand pharmaceutical storage conditions. Clinical trial products often require refrigeration between 2-8°C or freezing at -20°C or colder. Some specialized products demand storage at -80°C. Current electronic displays have limitations at extreme cold temperatures, making this a key technical challenge. The display must remain readable across the temperature range without drawing battery power.
Battery life presents both technical and sustainability challenges. Each label needs sufficient power for multiple content updates over the product’s shelf life, potentially 24-36 months. Single-use disposable batteries create environmental waste, while rechargeable batteries add complexity to the supply chain. The display must provide sufficient resolution for regulatory text requirements. Labels contain extensive information including drug name, lot number, expiration date, storage conditions, dosing instructions, and safety warnings, often in multiple languages.
Digital label systems must connect to existing pharmaceutical IT infrastructure, particularly enterprise resource planning and manufacturing execution systems. These systems already manage product data, lot tracking, and label content approval. Adding digital labels means creating interfaces that trigger label updates based on specific events like expiration date extensions or protocol assignments.
The software must maintain complete audit trails. Every label update needs documentation showing who authorized the change, what content changed, when the update occurred, and verification that the correct label received the update. This supports both regulatory compliance and product recall capabilities. Validation of the software follows strict protocols under 21 CFR Part 11 requirements for electronic records and signatures. The system must prevent unauthorized access, maintain data integrity, and provide secure authentication.
Near-field communication currently serves as the primary method for updating digital labels. NFC requires the label and reader device to be within a few centimeters of each other, providing security against unauthorized updates but limiting the ability to update multiple labels simultaneously. The communication method must work reliably in pharmaceutical environments where clinical sites may have limited WiFi coverage in storage areas and distribution centers handle thousands of products daily.
What are the Main Benefits of Digital Display Labeling in Pharma Clinical Trials
The advantages of digital display labeling extend beyond simple convenience, addressing fundamental challenges in clinical trial operations and patient safety.
Traditional paper label changes require four to six weeks from decision to implementation. Digital labels compress this to days or even hours. When a clinical trial needs to extend an expiration date, adjust dosing instructions, or correct label content, the speed of change directly impacts patient access to treatment. Faster changes mean fewer supply disruptions and reduced risk of patients discontinuing treatment due to product unavailability.
This speed advantage compounds across a product portfolio. Large pharmaceutical companies run hundreds of clinical trials simultaneously. Each trial may need multiple label updates during its lifetime. Reducing each change from weeks to days saves months of cumulative time across the development program.
Manual processes create opportunities for error. Printing labels requires correct template selection, accurate data entry, and proper material handling. Applying labels to products demands attention to placement, orientation, and adhesion. Each manual step introduces risk. One major pharmaceutical company reported that labeling-related deviations accounted for a significant portion of their quality events. Digital labels eliminate most manual steps. Content updates happen through system-controlled processes with built-in verification, reducing deviation rates and freeing quality resources for higher-value activities.
Clinical trials rarely proceed exactly as planned. Patient enrollment may accelerate or slow. Competing trials may create drug shortages. Protocol amendments may change dosing schedules. Paper labels lock in assumptions made during manufacturing, limiting flexibility to respond to changing conditions.
Digital labels enable dynamic supply chain management. Products can be reassigned between trials, shifted to different countries, or held for later use with minimal operational friction. This flexibility reduces the need to manufacture country-specific or trial-specific inventory, which often leads to waste when enrollment projections prove inaccurate. The ability to pool inventory across trials represents substantial value, reducing overall manufacturing requirements and minimizing waste from unused trial-specific products.
Clinical trial participants often manage complex dosing schedules with products requiring specific storage or handling. Traditional paper labels, especially multilingual booklets, can be difficult to read and interpret. Digital labels can adjust font sizes, display content in a patient’s preferred language, and present information across multiple pages rather than cramming everything into limited space. Navigation buttons allow patients to find specific information quickly. Additional safety features become possible with digital displays. Icons can supplement text instructions, providing visual cues for storage conditions or dosing times.
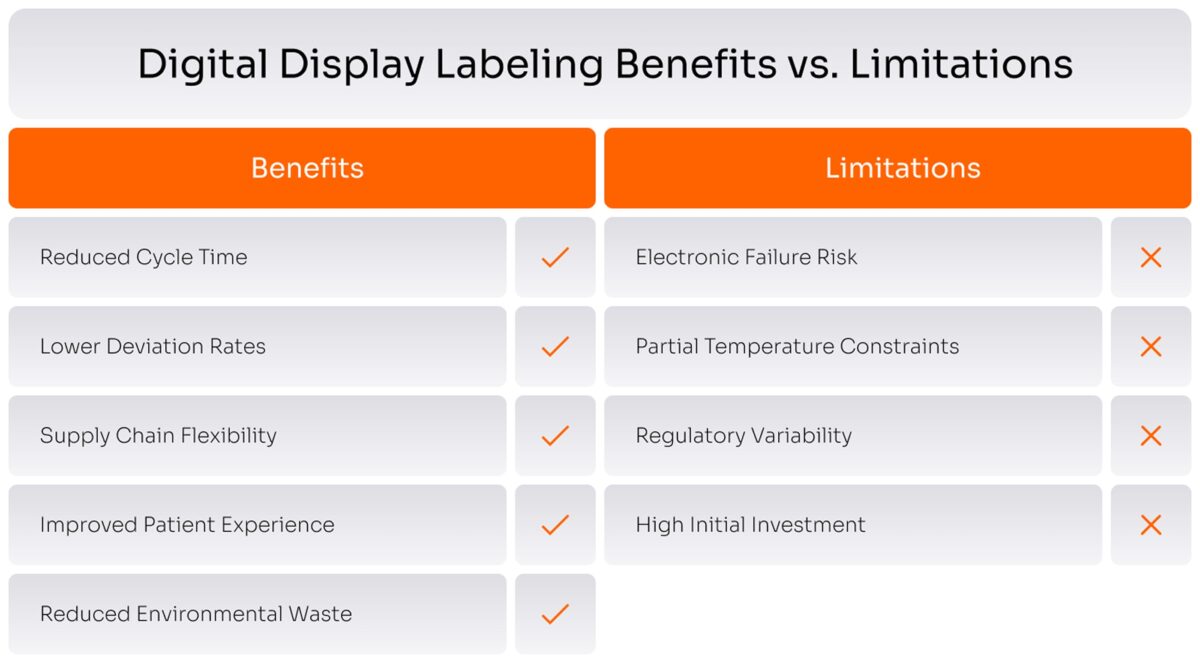
What are the Risks and Limitations of Digital Labels in Clinical Trials
Digital display labeling introduces new considerations that demand careful management and a clear-eyed assessment of trade-offs.
Electronic labels can fail in entirely different ways than their paper predecessors. Rather than fading or tearing, they succumb to battery depletion, display malfunction, or electronic damage. The remedies are straightforward but require discipline: battery monitoring systems with low-power alerts, ruggedized enclosures rated for pharmaceutical shipping abuse, and accelerated aging protocols that compress 18-24 months of real-world stress into weeks. Battery life must comfortably exceed product shelf life with margin for multiple updates.
Operating temperature limits pose a more intractable problem. Standard refrigeration at 2-8°C presents no difficulty, but specialized products requiring deep freezing at -80°C or cryogenic storage do. Most electronic components cease functioning below -20°C to -40°C—a significant constraint for cell and gene therapies. The pragmatic solution is hybrid labeling: deploy digital labels where conditions permit, revert to paper where they don’t. Alternatively, invest in specialized e-ink technologies engineered for extreme cold, though these remain nascent.
Health authorities have scant experience with digital labels, and global requirements vary wildly. Early engagement proves essential: pre-submission meetings with regulators, leveraging industry consortia like the Clinical Supply Leadership Forum to pool validation data, and designing systems flexible enough to display region-specific content from a validated core platform. Progress varies frustratingly by jurisdiction.
Reusability sounds environmentally virtuous until one confronts the logistics. Clinical trials ship globally; reverse logistics infrastructure doesn’t exist. Pilot programs partnering with existing clinical logistics providers offer a starting point. Establish regional processing hubs in trial-dense markets. Accept that single-use labels may be necessary initially, even as they increase electronic waste. Balancing sustainability with operational reality remains an unresolved tension.
Yalantis assists pharmaceutical companies of all sizes in navigating the complexities of digital display labeling, helping them overcome challenges such as battery life, temperature limitations, and regulatory uncertainty. We tailor investments to minimize the impact of these downsides, ensuring a robust and compliant transition to advanced labeling solutions.
How Long Does it Take to Implement Digital Display Labeling in Pharma Clinical Trials
Implementation timelines for digital display labeling depend heavily on organizational readiness, technical infrastructure, and regulatory strategy.
Companies beginning digital label programs typically spend six to twelve months evaluating hardware options, conducting feasibility studies, and developing initial prototypes. This phase includes testing different display technologies, battery configurations, and attachment methods on representative packaging formats. During prototyping, teams assess whether labels can withstand relevant storage conditions, maintain readability across temperature ranges, and survive shipping and handling.
Connecting digital label systems to existing pharmaceutical IT infrastructure represents the most time-intensive phase at twelve to eighteen months. Enterprise resource planning systems were built over years with complex data structures and validation requirements. Adding new interfaces requires careful design to maintain data integrity and system stability. Software validation under 21 CFR Part 11 and GxP requirements follows rigorous protocols covering all normal operations plus error conditions and edge cases. This validation cannot be rushed, as regulatory inspectors scrutinize software controlling critical manufacturing and distribution processes.
Even after technical readiness, companies typically run pilot studies before broad deployment, taking six to twelve months. These pilots test digital labels in real clinical settings with actual products and patients. Pilot programs validate operational procedures at clinical sites and provide evidence for regulatory submissions. Health authorities want data showing digital labels work in practice, not just in controlled tests.
Scaling from pilots to routine use across a clinical development portfolio takes additional time. Each new product format or packaging configuration may need specific label design. Most pharmaceutical companies implementing digital labels plan for two to three years from initial decision to widespread operational use. This timeline assumes adequate resources and management commitment.

How Much Does it Cost to Implement Digital Display Labeling in Pharma Clinical Trials
Cost analysis for digital display labeling must account for both upfront implementation expenses and ongoing operational savings to provide accurate total cost of ownership.
There is no single price tag on Digital Display Labeling in pharmaceutical operations. Assuming a case of an industry challenger who wants to stay competitive, we estimate the full implementation to form the next way:
Current digital label hardware costs approximately $10-50 per unit depending on specifications, volume, and feature set. Compare this to paper labels ranging from $0.10-2.00 per unit depending on complexity. The direct cost comparison appears unfavorable for digital labels. However, this ignores the costs of relabeling, which can run thousands of dollars per event when accounting for labor, materials, product handling, and supply chain disruption. A single avoided relabeling event can offset the premium for dozens of digital labels.
Software development, system integration, and validation represent substantial one-time costs, typically ranging from $500,000 to several million dollars depending on complexity and organizational IT maturity. These infrastructure costs are largely fixed, meaning they spread across the volume of products using digital labels. For companies with large clinical development portfolios, the per-product infrastructure cost becomes negligible.
Digital labels reduce costs in several areas that offset implementation expenses. Relabeling operations typically cost $10,000-50,000 per event when fully accounted for. Companies running large clinical programs may face dozens of relabeling events annually. Reduced deviation rates save quality resources, as each labeling deviation requires investigation, documentation, and often corrective action. Quality teams typically spend $5,000-20,000 per deviation in fully loaded costs. Waste reduction provides additional savings. Products that cannot be economically relabeled must be destroyed, losing the full manufacturing cost.
For a large pharmaceutical company with substantial clinical development activities, break-even on digital label investment typically occurs within 3-5 years. The business case strengthens for products with long clinical development timelines requiring likely expiration date extensions, global trials needing language flexibility, high manufacturing costs making waste reduction valuable, and complex supply chains where flexibility provides competitive advantage.
Again, this is not a final price tag, depending on your infrastructure it can still go up or down. Everything depends on the scale of your operations, amount of clinical trials and existing inventory management system.
For pharmaceutical companies that consider investing into Digital Display labeling, Yalantis offers a tripwire service of testing the digital display labeling implementation on a smaller scale to project main project outcome in the future. From initial assessment and hardware pick to building a dedicated tracking system, we help you to cover the whole transformation process.
Start with us to test the technology and project your future ROI
Ready to explore digital labeling without the full-scale investment?
Is Digital Display Labeling GxP and 21 CFR Part 11 Compliant?
Digital display labeling systems can meet Good Practice and regulatory requirements when properly designed, validated, and operated, though compliance is not inherent to the technology itself.
The FDA regulation 21 CFR Part 11 establishes standards for electronic records and electronic signatures. Digital labels create electronic records when content updates are documented in system logs. Systems must restrict access to authorized personnel only through user authentication. Audit trails must capture all system activity. When someone updates a digital label, the system records who made the change, what content changed, when the update occurred, and any approvals granted. These audit trails must be secure, timestamped, and cannot be deleted or modified.
Digital labels must align with GxP standards throughout the product lifecycle. Manufacturing controls ensure that digital labels are correctly applied to products during packaging. Distribution practices must maintain product integrity during shipping and storage. Clinical practices require that investigational products are properly identified, stored, and dispensed at clinical sites.
Validation demonstrates that the digital label system consistently produces expected results. The validation approach follows standard pharmaceutical industry practices with installation, operational, and performance qualification. Installation qualification verifies proper hardware and software setup. Operational qualification tests all system functions under controlled conditions. Performance qualification demonstrates sustained performance over time in real conditions.
Companies using digital labels must be prepared to explain and defend their systems during regulatory inspections. Documentation must clearly describe system architecture, data flows, validation protocols, and operational procedures. Batch records must show how digitally labeled products were manufactured, released, and distributed.
What is the Step-by-Step Implementation Algorithm for Digital Display Labeling in Clinical Trials?
Successful digital label implementation follows a structured approach that manages technical, operational, and organizational challenges systematically.
Step 1: Begin by identifying specific trials or products where digital labels provide clear value. Prioritize products with frequent relabeling needs, long development timelines, or complex supply chains. Quantify expected benefits including reduced cycle times, lower deviation rates, and decreased waste. Assess organizational readiness across IT infrastructure, quality systems, and operational capabilities. Secure executive sponsorship and adequate budget, as implementation requires sustained investment over multiple years.
Step 2: Evaluate available digital label hardware against requirements including display size and resolution, temperature range, battery life, attachment methods, and durability. Assess software vendors that provide label content management and update capabilities. Conduct pilot testing with shortlisted technologies by applying labels to representative packaging formats and exposing them to relevant storage conditions.
Step 3: Design system architecture showing how digital label systems connect to enterprise resource planning, manufacturing execution, and quality management systems. Develop functional specifications describing exactly what the system must do. Build and configure systems according to specifications. Create detailed test plans covering installation, operational, and performance qualification.
Step 4: Execute formal validation protocols following GxP requirements. Document validation results in a validation report and obtain quality assurance approval before moving to operational use. Prepare regulatory strategy for trials that will use digital labels. Engage health authorities early to discuss the technology and address questions.
Step 5: Revise standard operating procedures across manufacturing, quality, and supply chain functions. Train staff across all affected functions with hands-on practice. Prepare clinical site education materials with clear instructions explaining the digital label technology, how to verify content, and what to do if problems occur.
Step 6: Select a low-risk trial for initial implementation. Apply digital labels to a subset of clinical trial inventory and document the process. Execute at least one label update during the pilot, measuring actual cycle time and verifying audit trail capture. Collect feedback from all stakeholders and conduct a formal pilot review.
Step 7: Expand digital label use to additional trials and products based on pilot learnings. Monitor key performance indicators including label failure rates, update cycle times, deviation rates, and user satisfaction. Establish regular review cycles to assess performance and identify improvement opportunities. Plan for next-generation capabilities as technology matures.
From technology selection to GxP validation and training, we manage the entire end-to-end implementation so you can focus on your clinical trials.
Moving Forward with Digital Display Labeling for Pharma
The pharmaceutical industry stands at an inflection point. Paper-based processes that served adequately for decades now constrain the speed and flexibility needed for modern drug development. Digital Display Labeling offers a proven path forward, eliminating manual relabeling, reducing errors, and creating more agile supply chains.
Implementation requires significant upfront investment in technology, infrastructure, and organizational change. Companies must validate systems rigorously, train staff thoroughly, and engage regulators proactively. The timeline from decision to operational use typically spans two to three years with costs reaching several million dollars for large-scale deployments.
For pharma companies committed to accelerating patient access to new therapies while maintaining the highest quality standards, digital labels represent a strategic imperative rather than optional modernization. The technology is ready. The regulatory path is becoming clearer. The business case strengthens as implementation costs decline with broader industry adoption.
The question for pharmaceutical decision-makers is not whether to implement digital labels but when and how to begin. Companies starting now will gain experience, optimize processes, and establish competitive advantage in clinical supply operations. Patient access to innovative therapies depends on efficient clinical development. Every month saved in supply chain operations, every deviation prevented, every bit of waste eliminated contributes to faster, better drug development. Digital Display Labeling delivers these benefits at scale, making it essential infrastructure for the pharmaceutical industry’s future.