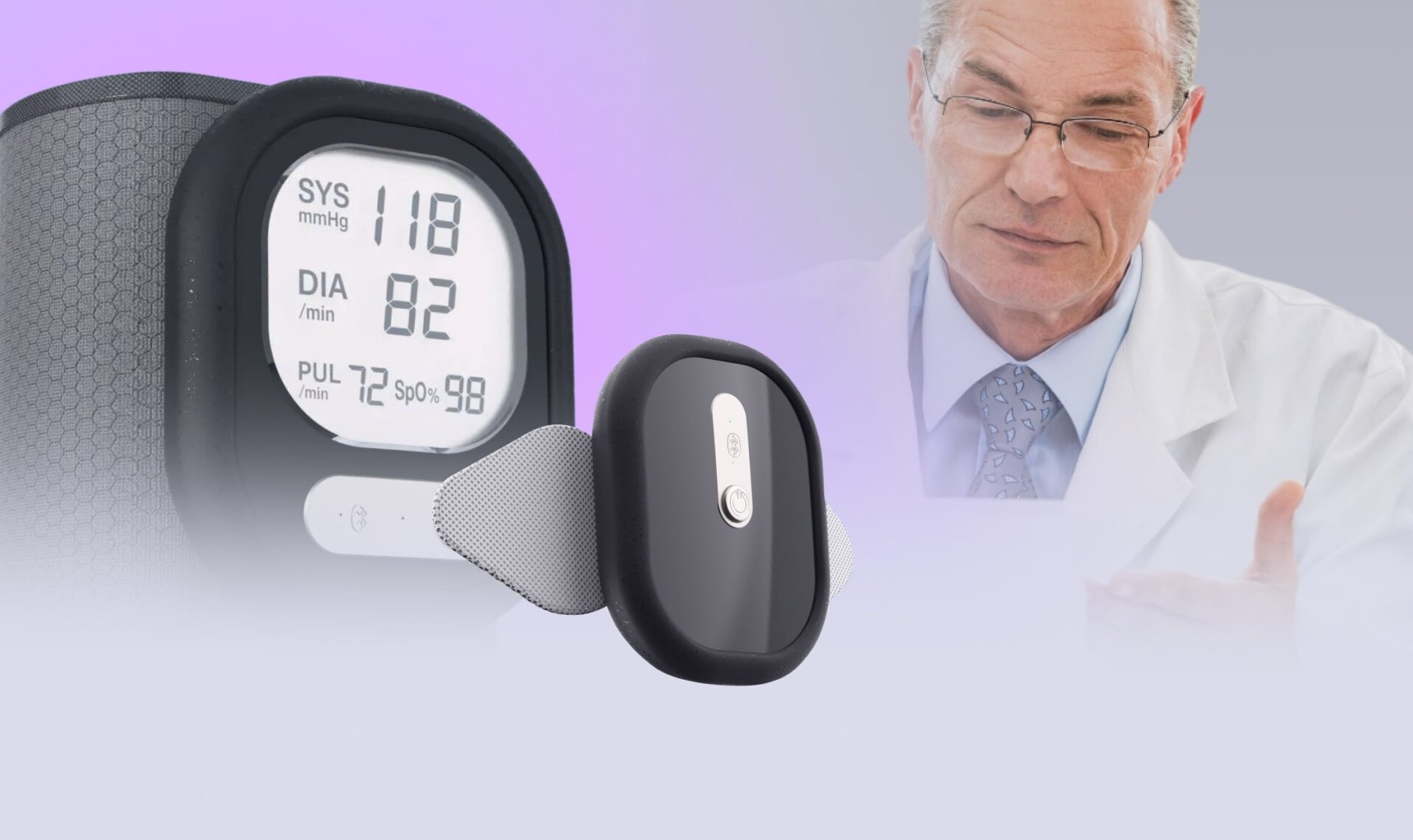
Build wearable devices for remote patient monitoring
Medical Device Prototyping and Healthcare App Development for Better Patient Data Connectivity
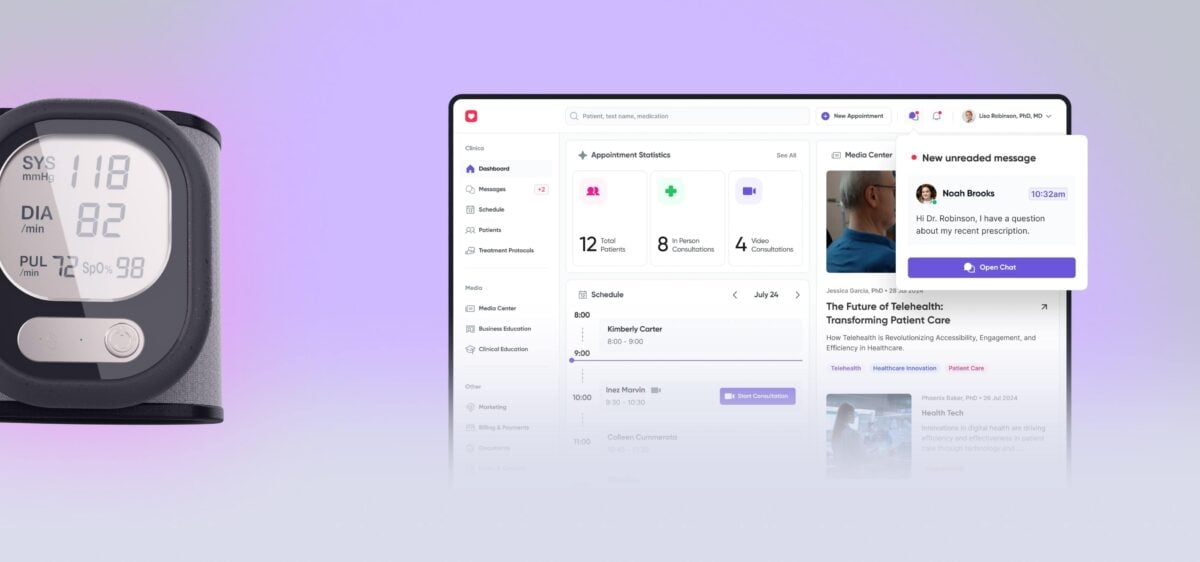
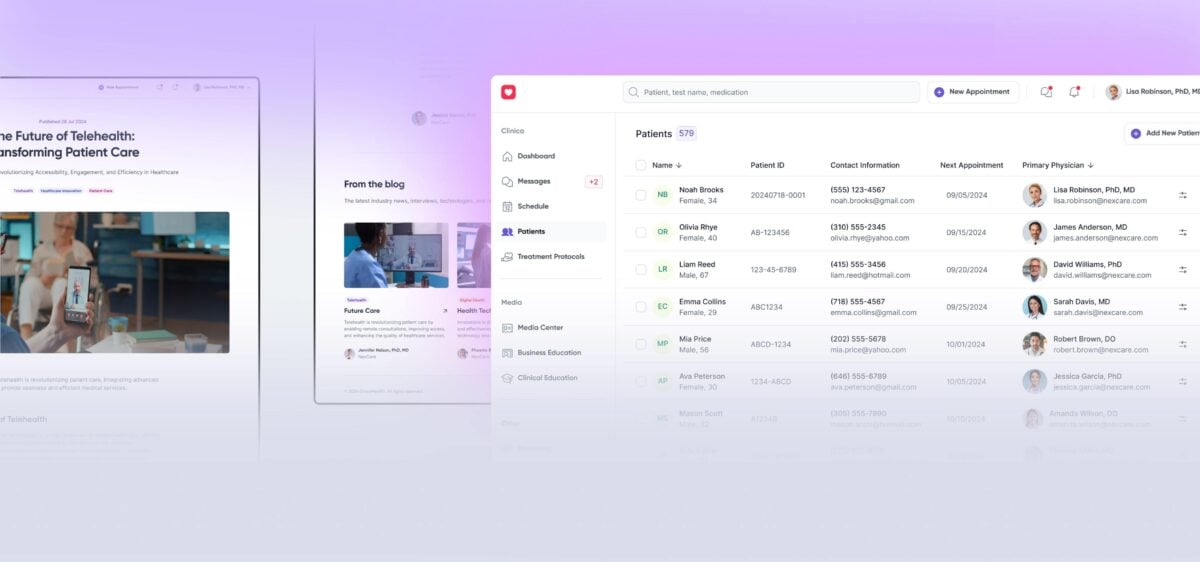
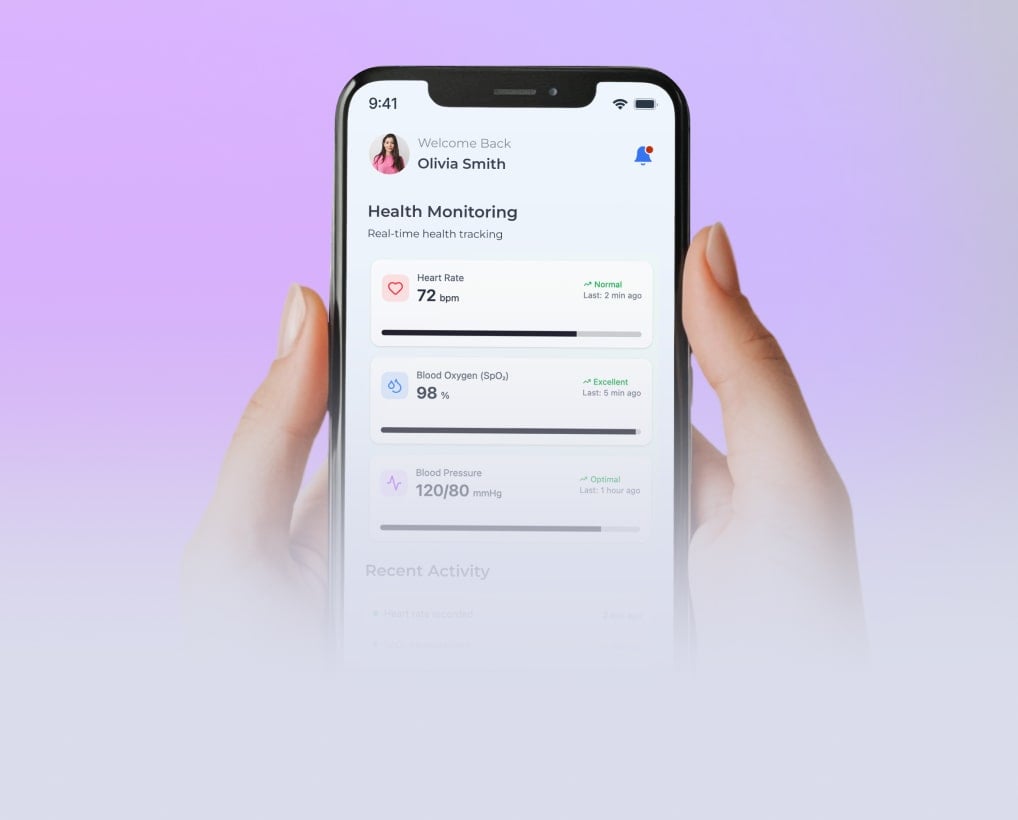
Following the client’s request for medical IoT device integration, Yalantis designed two wearable devices for ECG, oxygen, and blood pressure monitoring, developed a companion mobile app, and helped build a remote patient monitoring platform.
More clients attracted
Client engagement
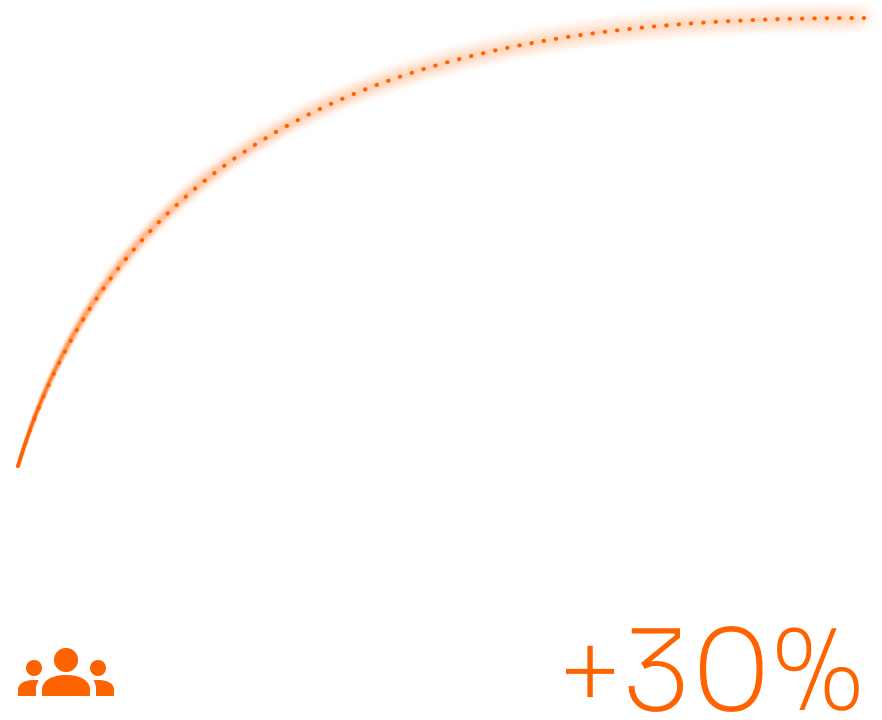
In patient user base
“Our client wanted to add wireless Remote patient monitoring to their hospital platform, so they asked us to develop medical devices and integrate them into their current system.”
– Mykhailo Maidan, CTO at Yalantis
Need an IoT app to match your devices? We can help.
Be our next success story. Share details about your project and book a call with us to discuss your goals.
From medical devices to industrial automation — we deliver complete enterprise solutions with regulatory compliance built-in. Everything under one roof.
Our offices
Poland
123 al. Jerozolimskie, Warsaw, 00-001
Ukraine
5 Dmytra Yavornytskoho Avenue, Dnipro, 49005
Cyprus
8 Athinon Street, Larnaca, 6023
Estonia
12 Parda, Tallinn, 10151
FAQ
-
How did you approach FDA/HIPAA?
Compliance started on day one: a secure Bluetooth communication layer was built to meet FDA Class I/II requirements, the FDA submission was supported through certification, and the mobile app was developed to be HIPAA-compliant.
-
How do you handle 24/7 data flow without blowing up costs?
Recent data stays in high-speed storage while older data is auto-archived to a cheaper tier; the pipeline runs on AWS (IoT Core, Data Streams, QuickSight) to keep ongoing costs down.
-
Can a solution like this plug into our hospitals’ EHRs if we have mixed HL7 versions?
Developing similar solutions, we add a compatibility layer that normalizes across HL7 versions so real-time patient data flows steadily into EHRs.
-
What does the patient/clinician experience look like?
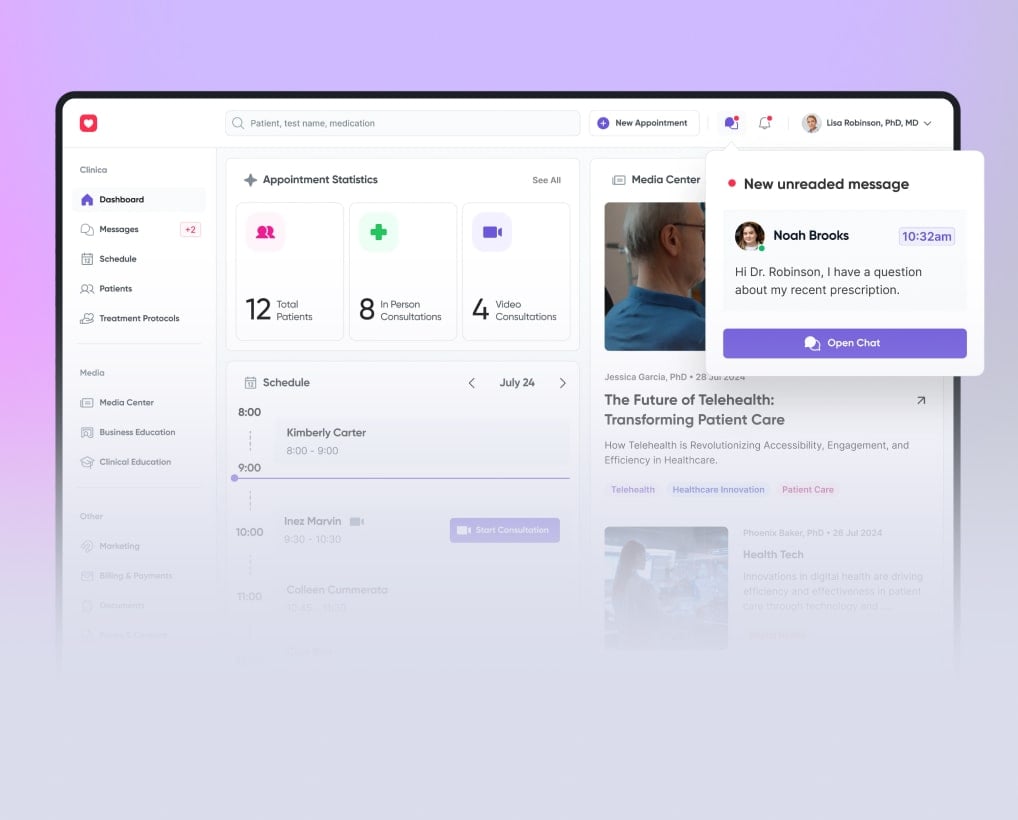
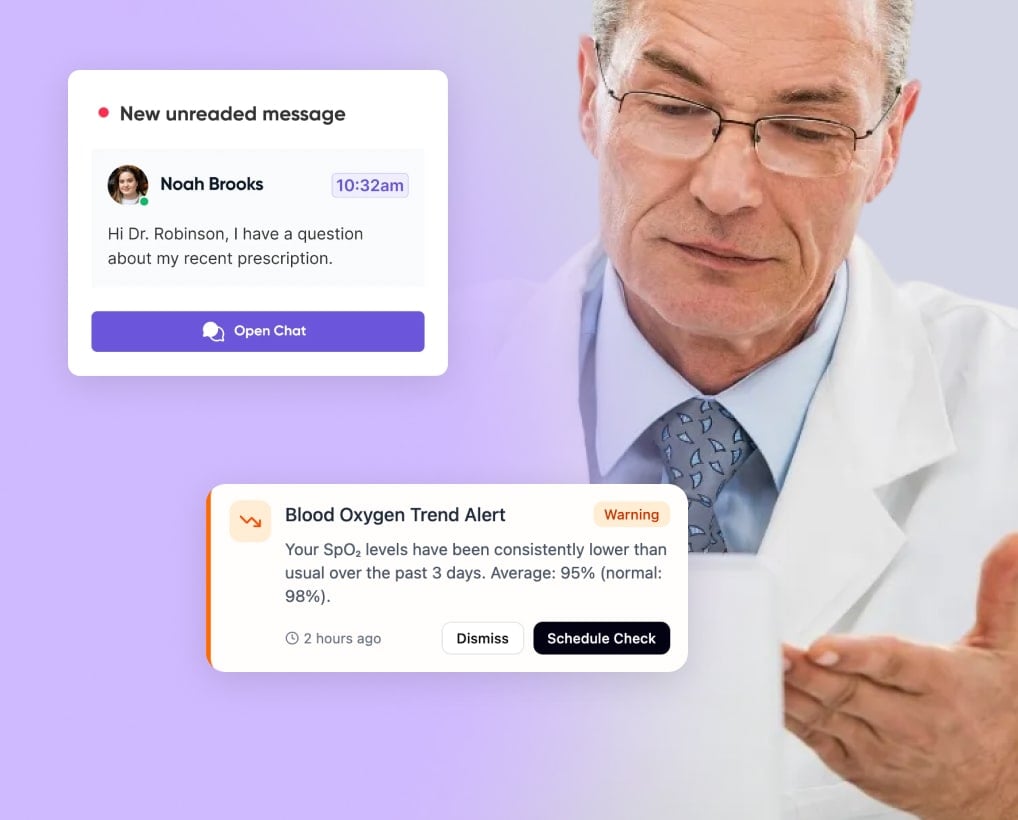
Patients see live vitals, alerts, and messages in the app; clinicians get dashboards inside the EHR with clear trends and alert markers for HR, SpO₂, and BP.
-
What business impact did the client report post-launch?
15% client growth, 20% revenue growth, and up to 30% higher engagement after rolling out the connected-care solution.
-
How did you sequence hardware and app work so the timeline didn’t stall?
Our team designed the wearable ECG device and SpO₂/BP monitoring devices first through comprehensive wearable cardiac devices development. Then while the devices went into manufacturing we built the HIPAA-compliant app and accessible UI in parallel to keep delivery moving.
Let’s Start from call scheduling
- Schedule a call
- We collect your requirements
- We offer a solution
- We succeed together!
Thank you for contacting us.
Meanwhile, you can explore our hottest case studies and read
client feedback on Clutch.
We are open for partnerships too
Check out our refferal program. Find out all benefits.