Digital Labeling Services
for Pharma
A validated, end-to-end service to eliminate paper-based risks and create a fully digital, GxP-compliant labeling process. We are a technical partner for Heads of Quality, Operations, and Clinical Supply frustrated with the constant deviations, manual errors, and compliance risks of traditional paper labels.
Challenges Our Digital Displays Solve
-
Costly deviations and CAPAs from manual label errors
Instead of managing a stream of deviations from incorrect printing, application, or transcription errors, our service implements a fully system-controlled process. This eliminates the human error inherent in manual, paper-based workflows and the associated risk to patient safety.
-
Risky and cumbersome manual relabeling for updates
Forget the operational nightmare of recalling products to a depot for manual relabeling just to update an expiry date. Our service validates a digital process that allows you to update label content instantly and remotely, reducing relabeling lead time from months to a single day.
-
Product loss from physically damaged or illegible labels
Paper labels are easily compromised by moisture, cryogenic storage, or simple abrasion, leading to faded ink, flaking, or labels falling off entirely. This can render a high-value IMP unsaleable. We qualify hardware designed to withstand the rigors of the clinical supply chain.
-
Lack of GxP-compliant traceability for label changes
Manual relabeling processes are not inherently traceable, creating a significant compliance gap and risk during audits. Our validated, 21 CFR Part 11 compliant system provides an unalterable audit trail for every single label update, ensuring you are audit-proof from day one.
-
Illegible text and complex, multi-language booklet labels
Our DDL solution offers a clear e-paper screen, eliminating the need to decipher small fonts on vials or lengthy booklets. This technology provides adjustable font sizes and easy page-flipping, ensuring critical information is always legible and simple to navigate.
-
Inflexible inventory and high waste from static, protocol-specific labels
Paper labels are static and must be pre-printed for specific protocols and countries, leading to long lead times and high waste. Our DDL platform lets you use a single, protocol-agnostic batch and update the label digitally just before dispensing, slashing both waste and lead times.
Yalantis Digital Display Labeling Services
-
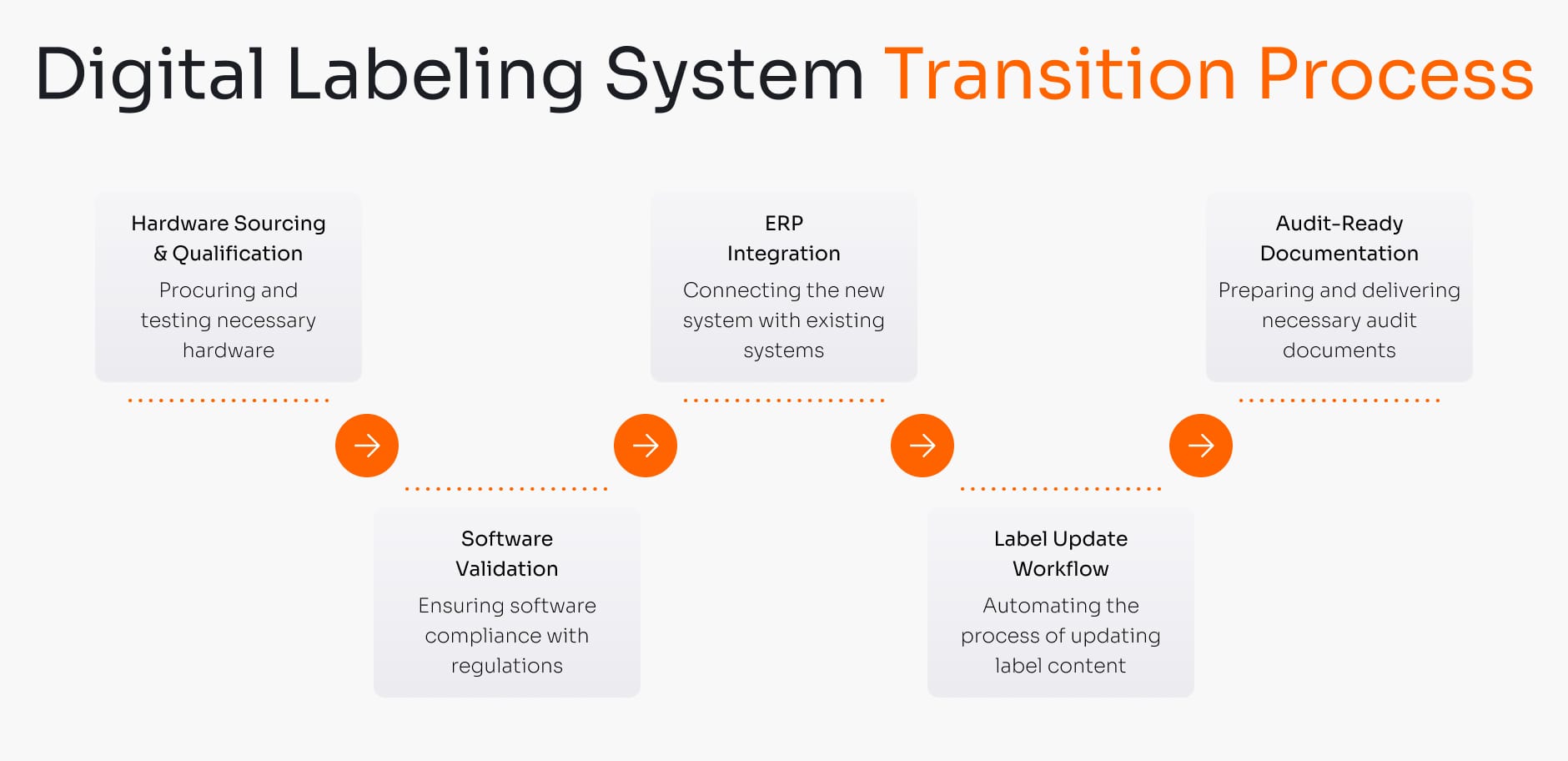
End-to-End Digital Display Labeling Implementation
A fully managed, turnkey service to transition your clinical supply chain from paper to a fully validated, GxP-compliant digital labeling platform. We manage every step, from hardware sourcing and qualification to 21 CFR Part 11 software validation and ERP integration, delivering an audit-proof system.
Key Features:
- Comprehensive GxP Validation Support
End-to-end system validation based on GAMP 5 principles, ensuring full 21 CFR Part 11 compliance for all electronic records and signatures. - Hardware IQ/OQ Execution & Documentation
Full Installation Qualification (IQ) and Operational Qualification (OQ) for all DDL hardware, including environmental and IMP compatibility testing. - Validated ERP/WMS Integration
GxP-validated API development to connect your core ERP, WMS, or MES systems directly to the DDL platform for a single source of truth - Automated Label Update Workflow
A system-controlled, validated process for instantly updating label content (e.g., expiry dates, protocol numbers) with full, traceable confirmation. - Comprehensive Validation Documentation Package
Complete handover of all GxP documentation, including your Validation Master Plan (VMP) and a final Validation Summary Report for your QMS.
- Comprehensive GxP Validation Support
-
GAMP 5 Validation & 21 CFR Part 11 Compliance-as-a-Service
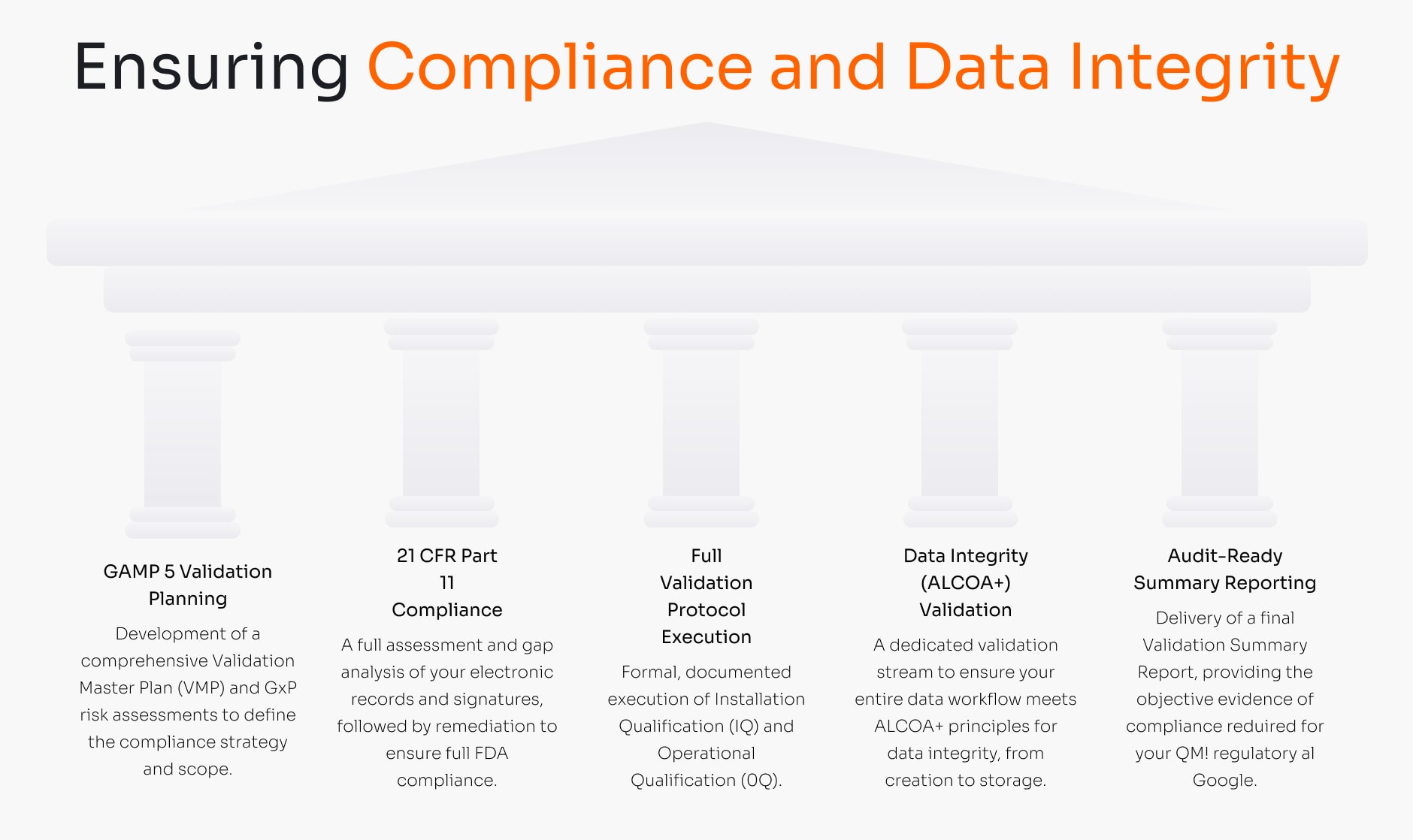
A dedicated compliance service for clients who have a DDL system but lack the in-house GxP expertise to validate it. We ensure your entire process, from the ERP data source to the e-paper display, is fully compliant, data-secure, and ready to face FDA or EMA scrutiny.
Key Service Deliverables:
- GAMP 5 Validation Planning
Development of a comprehensive Validation Master Plan (VMP) and GxP risk assessments to define the compliance strategy and scope. - 21 CFR Part 11 Compliance
A full assessment and gap analysis of your electronic records and signatures, followed by remediation to ensure full FDA compliance. - Full Validation Protocol Execution
Formal, documented execution of Installation Qualification (IQ) and Operational Qualification (OQ). - Data Integrity (ALCOA+) Validation
A dedicated validation stream to ensure your entire data workflow meets ALCOA+ principles for data integrity, from creation to storage. - Audit-Ready Summary Reporting
Delivery of a final Validation Summary Report, providing the objective evidence of compliance required for your QMS and regulatory audits.
- GAMP 5 Validation Planning
-
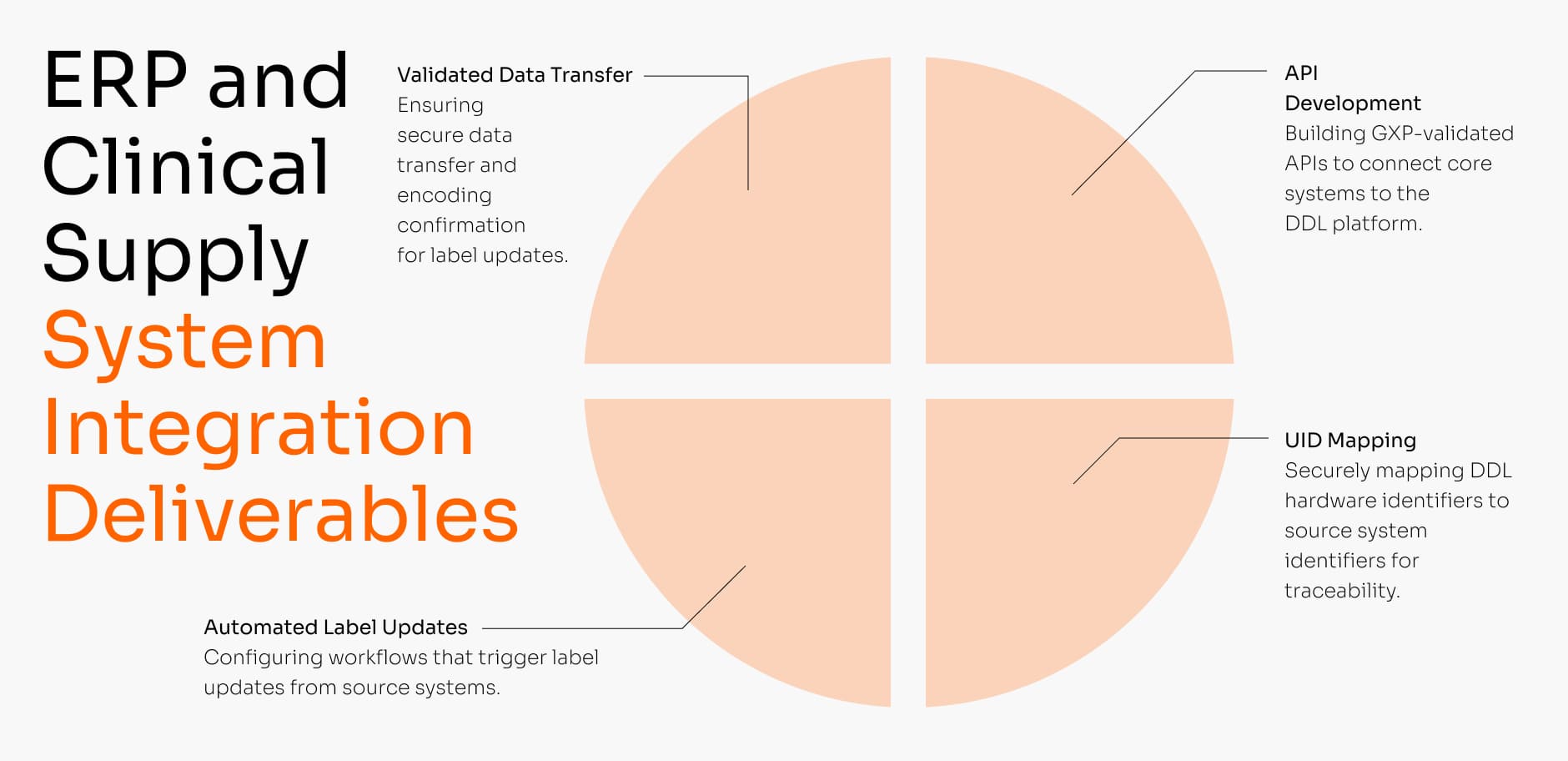
ERP & Clinical Supply System Integration
A specialized data engineering service designed to connect your fragmented IT landscape. We build the robust, GxP-validated integrations that link your core clinical supply or ERP system (e.g., SAP) directly to the DDL platform, enabling a fully automated, system-controlled labeling process.
Key Service Deliverables:
- GxP-Validated API Development
Building robust, GxP-validated APIs to connect your core ERP, WMS, or MES systems directly to the DDL platform. - Unique Identifier (UID) Mapping
Securely mapping unique DDL hardware identifiers (like NFC UID) to your source system identifiers (like ERP ID) for 1:1 traceability. - Automated Label Update Triggers
Configuring automated workflows that trigger label updates (like expiry date extensions ) directly from your source ERP or clinical supply system. - Validated Data Transfer & Confirmation
Ensuring secure, GxP-validated data transfer and “positive encoding confirmation” to verify the correct label was updated successfully.
- GxP-Validated API Development
-
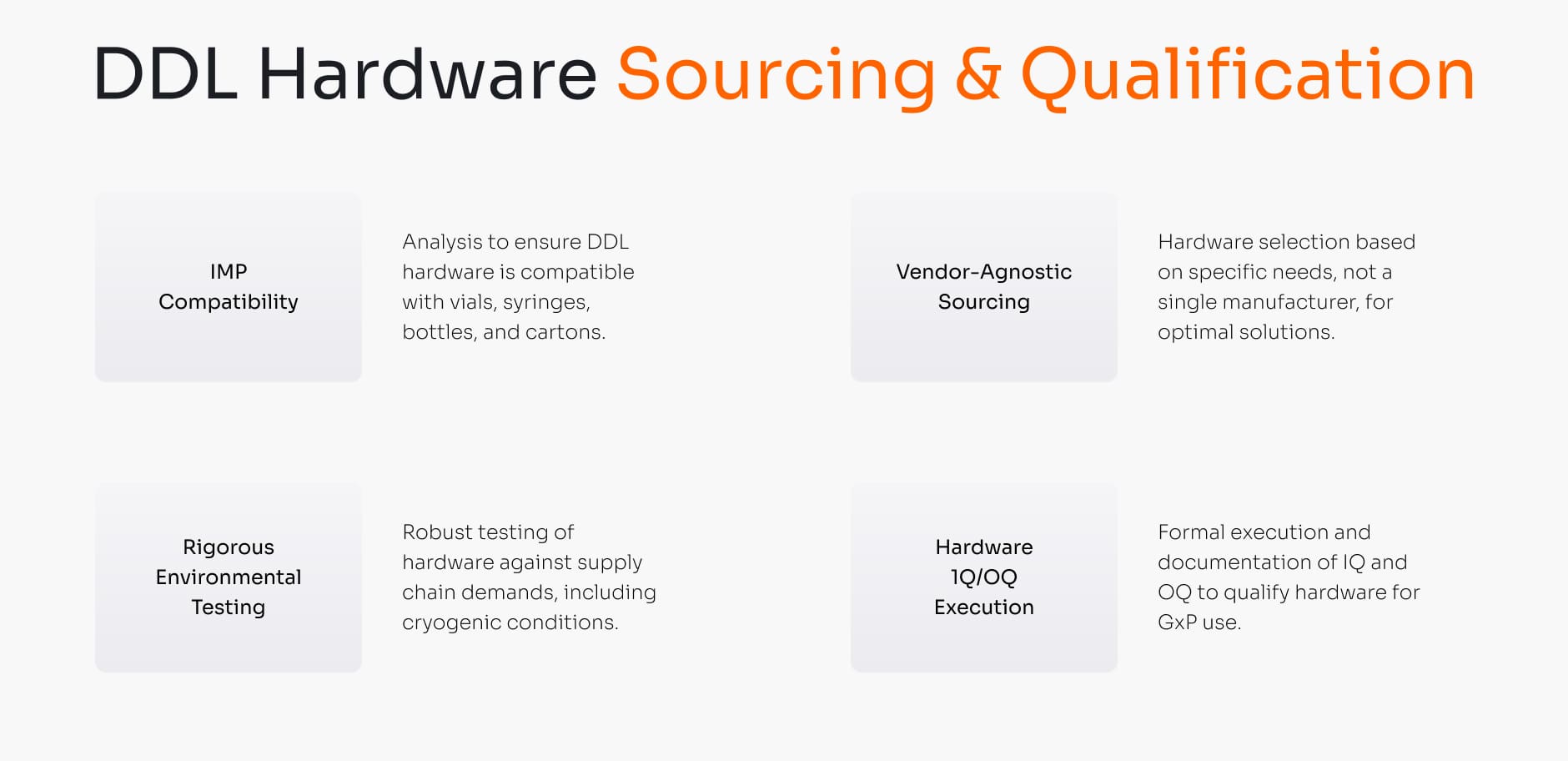
DDL Hardware Sourcing & Qualification
A consultative engineering service to solve the physical challenges of DDL implementation. We analyze your specific IMPs (vials, syringes, bottles) and source, test, and qualify the optimal hardware, ensuring it meets the rigorous demands of your cold chain and clinical sites.
Key Service Deliverables:
- IMP & Packaging Compatibility
Analysis and assessment to ensure DDL hardware is fully compatible with your specific IMPs, including vials, syringes, bottles, and cartons. - Vendor-Agnostic Sourcing
Hardware selection and sourcing based on your specific needs, not a single manufacturer, to find the optimal and most cost-effective solution. - Rigorous Environmental Testing
Robust testing of hardware against the rigorous demands of your supply chain, including functionality in cryogenic and ultra-low temperature conditions. - Hardware IQ/OQ Execution
Formal execution and documentation of Installation Qualification (IQ) and Operational Qualification (OQ) to qualify the DDL hardware for GxP use.
- IMP & Packaging Compatibility
-
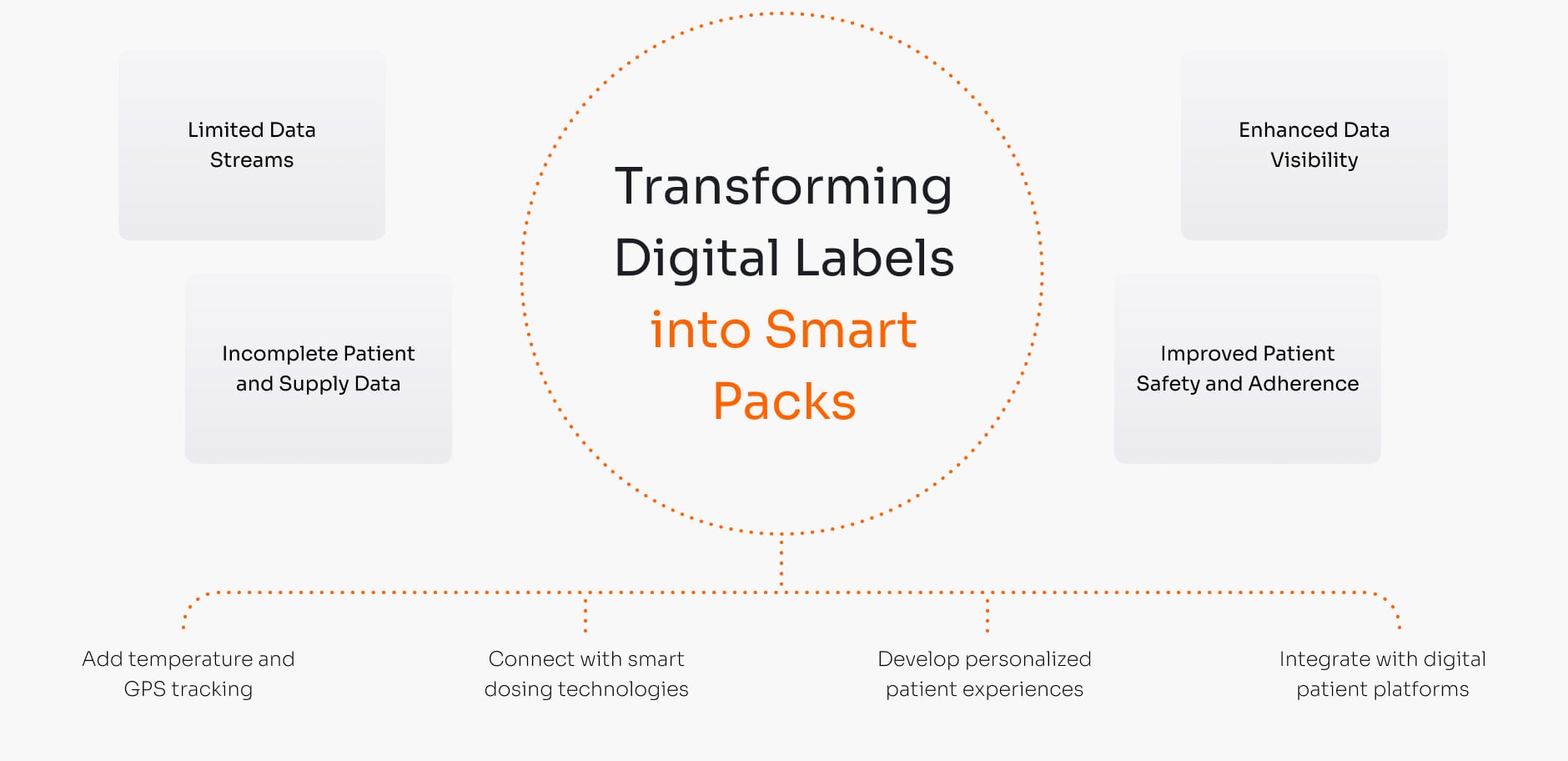
“Smart Pack” IoT & Adherence Module Development
Evolve your DDL from a digital label to a “Prototype 4” smart pack. This service adds advanced IoT capabilities to your platform, providing new data streams to enhance patient safety, adherence, and supply chain visibility.
Key Service Deliverables:
- Integrated IoT Tracking
Integrates temperature monitoring and geolocation (GPS) into the DDL platform, evolving it into a “Prototype 4” smart pack. - Smart Dosing & Adherence
Connects the platform with smart dosing technologies to accurately track, monitor, and improve medication adherence. - Patient-Centric Features
Develops personalized patient experiences, including visit schedules, dosing reminders, and motivational messages to encourage therapy continuation. - Digital Engagement Platform
Integrates the DDL with digital patient platforms, enabling features like patient diaries and two-way communication channels with clinical sites.
- Integrated IoT Tracking
Our success stories
Benefits of a Validated Digital Display Labeling
-
Drastically Reduce Labeling Deviations
By replacing manual, error-prone paper processes with a system-controlled, validated workflow, you eliminate the primary source of label-related deviations, rework, and CAPAs.
-
Maximize Operational & Supply Chain Flexibility
Instantly update expiry dates, add new languages for different countries, or re-assign pooled inventory to different trials. This agility reduces product waste and shortens critical lead times, making your supply chain more resilient.
-
Create a Modern, Defensible, and Compliant Supply Chain
Our service delivers exactly what auditors want to see: a modern, 21 CFR Part 11 compliant system. You move away from untraceable manual steps to a robust process that provides unalterable evidence of your label’s content at every point in its journey.
-
Enhance Patient Safety and Site Experience
DDLs provide clear, legible information, with the ability to adjust font size or display information in a patient’s preferred language. This improves the user experience, reduces the administrative burden on clinical site staff, and enhances overall patient safety and adherence.
-
Streamline Initial Label Creation and Production
Our service streamlines initial label creation and production by replacing the labor-intensive process of designing, printing, and delivering complex paper booklet labels with an agile, digital workflow, significantly reducing the timeline and effort for initial label production.
-
Enterprise-Grade Security & Scalability
Our validated process streamlines labeling and distribution, eliminating paper’s long lead times and expediting supply operations. This reduces supply delays and shortages, contributing to faster drug development and quicker patient access to life-saving treatments.
Testimonials from our clients
FAQ
What is the difference between your service and just buying DDL hardware?
Hardware is just a component; a validated process is the solution. Our service is the critical GxP bridge. We manage the entire validation lifecycle (IQ/OQ/PQ) and ensure the end-to-end software process, from your ERP to the DDL system, is 21 CFR Part 11 compliant. Buying hardware leaves the entire integration, validation, and compliance burden on your internal team.
How do DDLs handle ultra-low temperature storage?
This is a known challenge for electronics. Our hardware selection process is critical here. While most e-paper displays cannot be updated in frozen conditions, the information remains perfectly legible. The update (e.g., via NFC) is performed at a qualified point in the process, such as during packing or just before dispensing, in a system-controlled and validated manner.
How do you validate the “right” update goes to the “right” label?
This is the core of our software validation. The system maps unique identifiers. Each DDL has a unique, unchangeable ID (like an NFC UID). The update order from your ERP is linked to this specific ID. The system requires a “positive encoding confirmation” to verify that the DDL was updated correctly, creating a closed-loop, documented process.
What about sustainability? Aren’t electronics more wasteful than paper?
While DDLs involve electronics, the waste from traditional labeling is significant, including label re-work, wasted inventory from label errors, and entire shipments being discarded. By enabling inventory pooling and eliminating re-work, DDLs can dramatically reduce overall product waste. Our service also focuses on sourcing reusable DDLs to create a circular model, further improving the sustainability case.
Are health authorities like the FDA & EMA actually accepting digital labels instead of paper?
Yes, because unlike past “e-label” concepts that relied on QR codes, our DDL solution displays all required regulatory content as human-readable text directly on the label itself. Health authority concerns were focused on solutions where the required text was not visible on the product. Our fully validated, 21 CFR Part 11 compliant process ensures the method of displaying and updating this digital text is secure, traceable, and defensible.
Contact us

got it!
Keep an eye on your inbox. We’ll be in touch shortly
Meanwhile, you can explore our hottest case studies and read
client feedback on Clutch.

Nick Orlov
IoT advisor
How to get started with IoT development
-
Get on a call with our Internet of Things product design experts.
-
Tell us about your current challenges and ideas.
-
We’ll prepare a detailed estimate and a business offer.
-
If everything works for you, we start achieving your goals!