Pharma Software Development and Hardware Design
GxP-compliant services that transform disconnected data into a single source of truth. Engineered to ensure compliance and provide the audit-proof evidence your quality system demands.
Pharma & Life Sciences challenges we solve
-
Manual labeling as a primary source of deviations and costly CAPAs
Intelligent, digital labeling solutions will help you eliminate human error, dynamically update expiry dates, and avoid the immense risk and cost of manual relabeling campaigns.
-
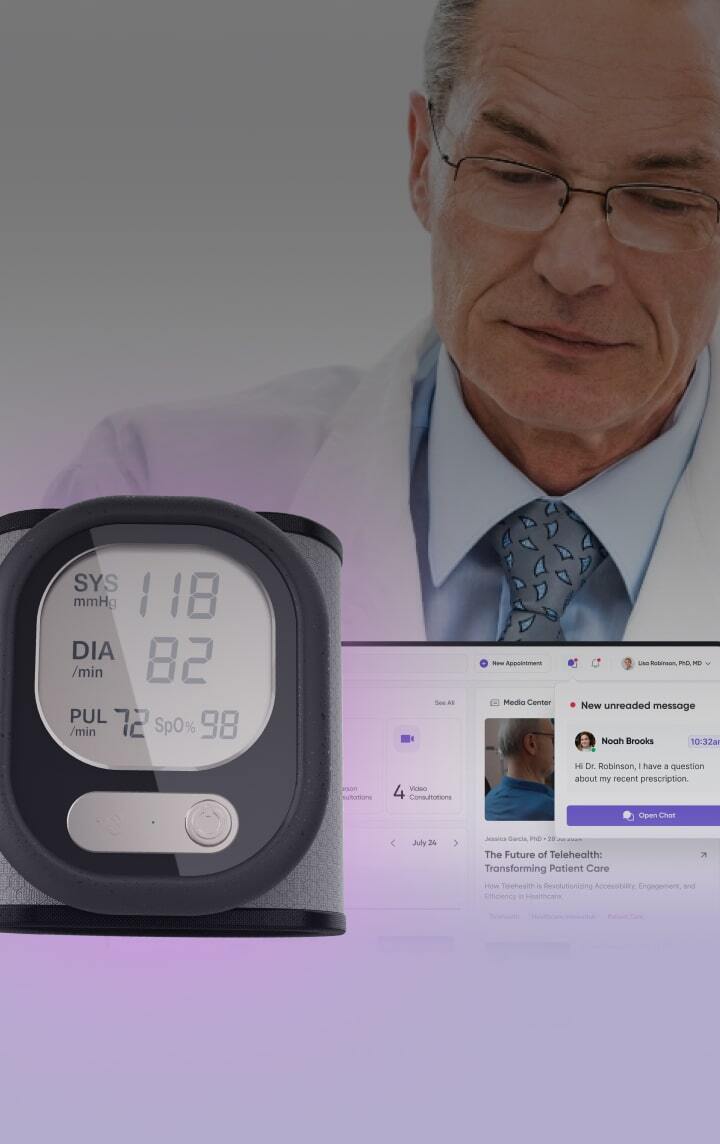
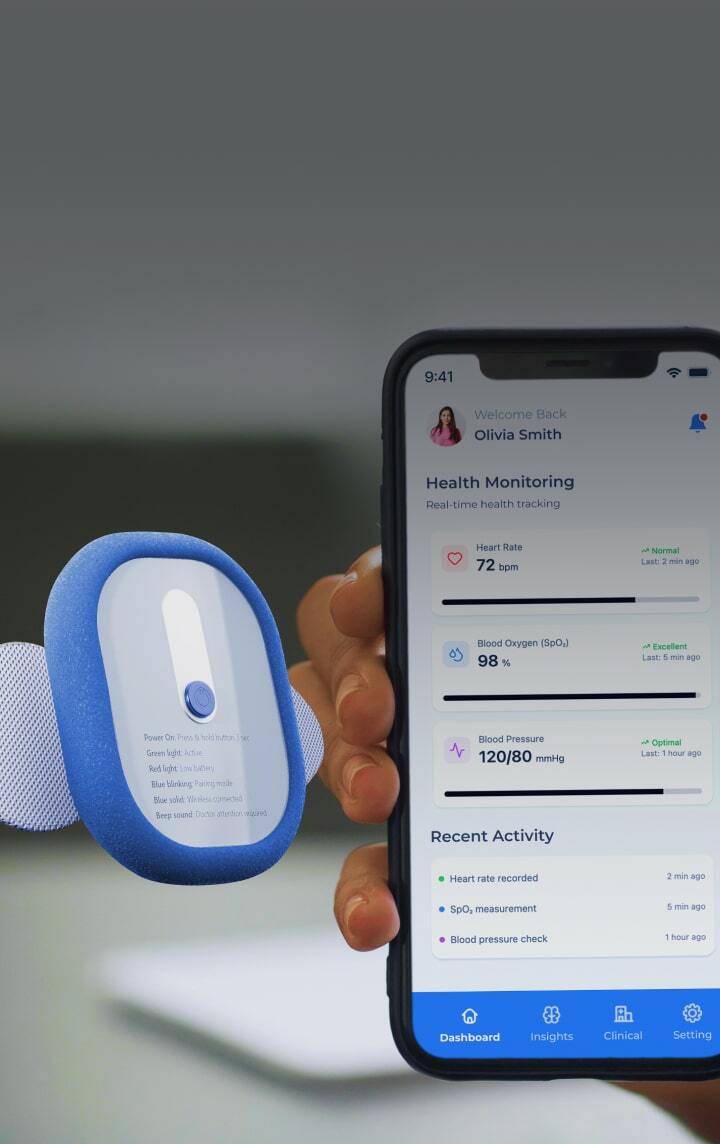
Cold chain integrity relies on after-the-fact data loggers
Instead of discovering a multi-million dollar product loss days after it occurs, our solutions provide continuous, real-time monitoring and alerting, giving you the validated data to prove your cold chain was never broken.
-
“Chain of Identity” for cell therapies is a life-or-death manual process
Forget the immense risk of paper-based verification. We deliver an unbroken, “vein-to-vein” digital record that binds a patient’s identity to their specific therapy at every critical handover point, ensuring absolute certainty.
-
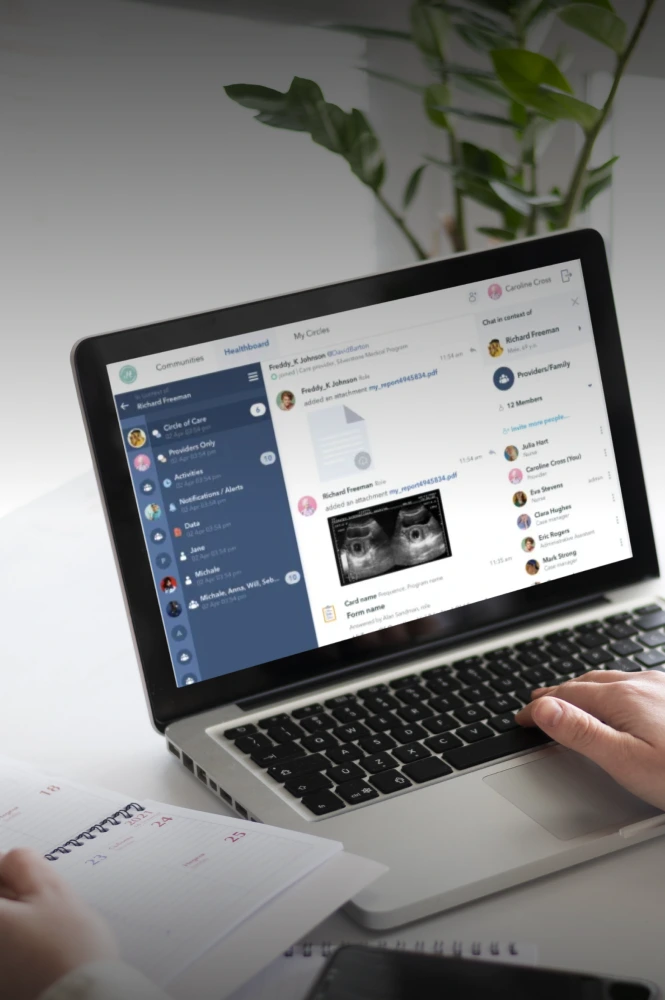
Disconnected ERP, QMS, and 3PL systems create compliance blind spots
You can prevent audit failures and data chaos by connecting these disparate platforms into a cohesive, validated ecosystem that provides a single, unalterable history for every batch.
-
Supply chain insecurity threatens patient safety and brand reputation
The constant threat of product theft, diversion, and counterfeit infiltration requires a new level of defense. We provide unit-level traceability solutions that create an unbreachable digital audit trail from the factory to the final dispense.
-
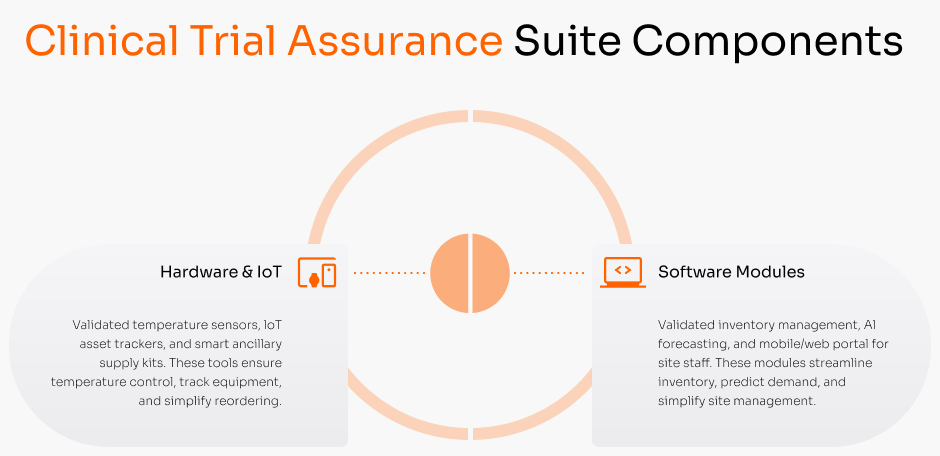
Clinical trial logistics complexity is slowing down research
Managing investigational medicinal products (IMPs) and patient biospecimens across global sites is a logistical nightmare. Our solutions provide a central, compliant platform to manage inventory, track samples, and accelerate trial timelines.
GxP-Compliant Services for Pharmaceuticals and Life Sciences
-
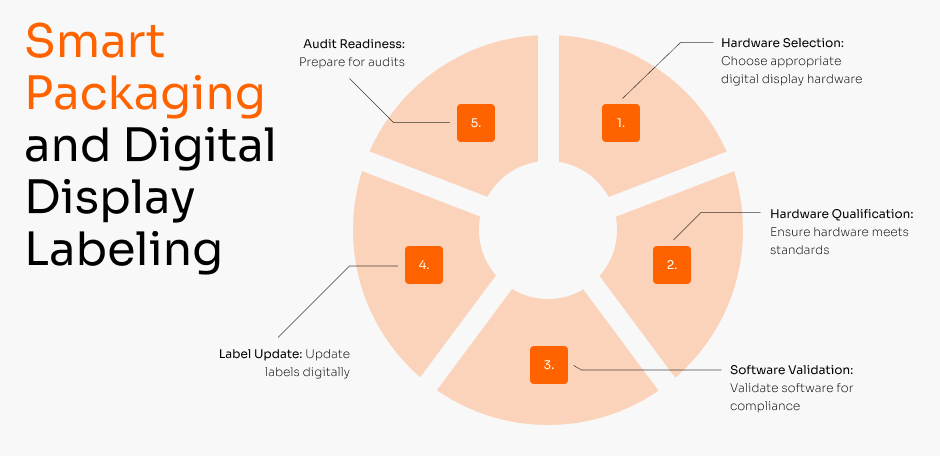
Smart packaging and Digital Display Labeling
Learn moreA validated, end-to-end service to eliminate paper-based risks and create a fully digital, GxP-compliant labeling process. We manage the entire transition, from hardware selection and qualification to the full validation of the 21 CFR Part 11 compliant software, ensuring your new paperless system is audit-proof from day one.
-
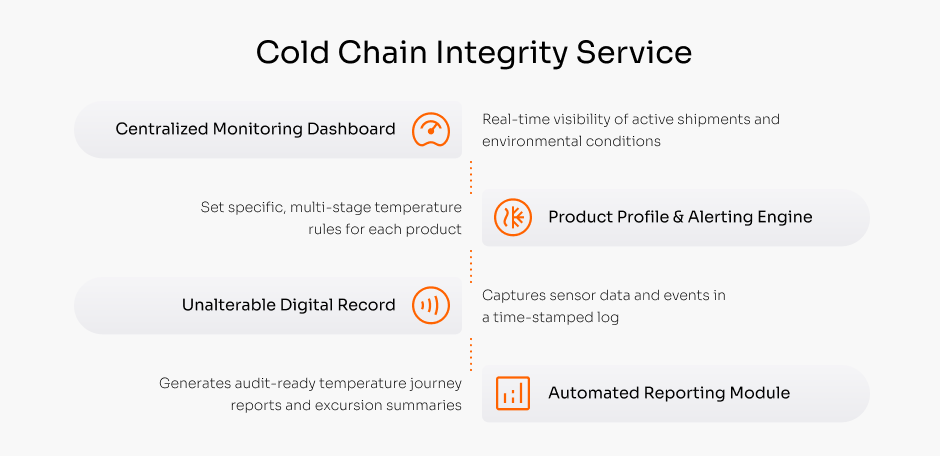
Cold Chain Integrity Service
Learn moreAn integrated hardware and software solution that provides the unalterable scientific evidence of your product’s complete temperature journey. We deliver the validated, audit-proof record you need to protect your brand and ensure patient safety.
-
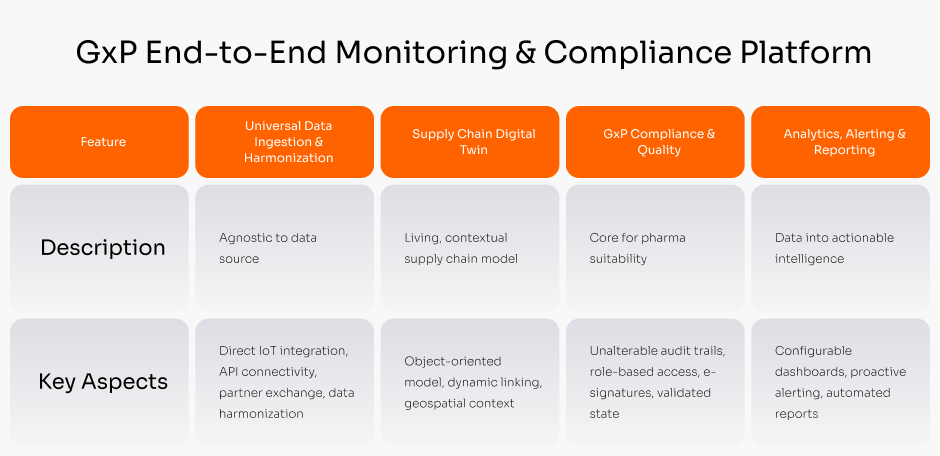
Pharma Supply Chain Data Governance
A fixed-fee service to manage the implementation and validation of new GxP-compliant supply chain systems, ensuring on-time, on-budget, and audit-proof deployments.
What’s included:
- GxP Validation Accelerator Pack
A comprehensive suite of pre-built Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) templates to streamline system validation. - Dedicated Validation & Governance Team
Acts as a direct extension of your internal team, providing specialized expertise to manage the entire data governance framework and validation lifecycle. - Legacy System Integration
A proven methodology for creating robust and reliable integrations between the new platform and your existing legacy systems, such as ERPs and Quality Management Systems (QMS). - Audit-Ready Documentation
A complete, audit-proof documentation package, including the data governance framework and all executed validation scripts, ensuring regulatory readiness from day one.
- GxP Validation Accelerator Pack
Our success stories
Why Partner With Us?
-
GxP Is Our First Language
We are not a generic IT vendor. We are compliance and quality experts who build technology. We understand GAMP 5, 21 CFR Part 11, and the unforgiving demands of a validated state.
-
Full-Stack, Validated Delivery
From GxP-compliant firmware on our custom sensors to a fully validated cloud platform, we deliver a complete, integrated, and audit-ready solution under one roof.
-
Uncompromising Data Integrity
Our systems are architected around ALCOA+ principles. We provide the unalterable audit trails and electronic records that are mandatory to satisfy FDA and EMA inspections.
-
Built for the Long Haul
We understand the long lifecycle of pharmaceutical operations. We provide the robust change control, long-term support, and deep domain expertise you need in a strategic partner.
-
Problem-Solving Approach
We don’t sell tech modules; we sell quantifiable answers to your most expensive problems. Our process begins with a deep discovery phase to understand your challenges.
-
Enterprise-Grade Security & Scalability
Our solutions are architected for the demands of global pharmaceutical enterprises. We implement a security-first design from day one and ensure our platforms can scale reliably as your clinical trials or commercial operations grow.
Testimonials from our clients
FAQ
Why choose your custom solution over an off-the-shelf GxP product?
Off-the-shelf software forces you to adapt your validated processes to its rigid limitations. We build solutions around your specific SOPs and quality systems, ensuring a perfect fit that provides a defensible, compliant advantage that generic software cannot.
How do you handle system validation?
Validation is not an add-on; it’s central to our methodology. We provide a comprehensive “Validation Accelerator Pack” with IQ/OQ/PQ templates and work as an extension of your quality team to ensure a smooth, compliant, and successful deployment.
Our company uses multiple legacy systems. Can you integrate with them?
Yes. A core competency is creating robust, validated data pipelines that unify your critical data from ERP, QMS, and partner systems into a single, reliable platform, eliminating silos and ensuring end-to-end data integrity.
What makes your IoT hardware GxP-ready?
Our hardware is purpose-built for pharmaceutical use. We manage the entire lifecycle, from designing secure firmware to providing certified, NIST-traceable calibration for all sensors. Our devices are engineered for extreme reliability, from standard cold chain to cryogenic temperatures.
How do you manage change control for software and firmware updates?
We treat the validated state with the seriousness it deserves. All updates follow a rigorous, documented change control process. For firmware, we manage fleet-wide rollouts in a controlled manner that ensures devices are updated without compromising ongoing shipments or creating a compliance crisis.
Can your solutions support decentralized clinical trials (DCTs)?
Absolutely. Our platforms are designed for the “last-mile frontier.” We provide validated, kit-level temperature sensors and simple mobile portals for clinical site staff and even patients, solving the challenges of GxP compliance in a patient’s home.
Who are the key stakeholders we need to involve for a successful project?
A successful deployment requires buy-in from a trinity of leaders. We partner closely with the Operational Owner who feels the daily pain, the Quality Gatekeeper who can kill any deal that compromises compliance, and the IT Validator who must integrate and maintain the system.
Contact us

got it!
Keep an eye on your inbox. We’ll be in touch shortly
Meanwhile, you can explore our hottest case studies and read
client feedback on Clutch.

Nick Orlov
IoT adviser
How to get started with IoT development
-
Get on a call with our Internet of Things product design experts.
-
Tell us about your current challenges and ideas.
-
We’ll prepare a detailed estimate and a business offer.
-
If everything works for you, we start achieving your goals!