Let’s model a situation: your Phase III trial just received regulatory approval to expand into Japan and South Korea. You have sites ready to activate, patients waiting to enroll, and clinical teams prepared to begin. But your supply chain cannot deliver products for another 14 weeks because you need to design new labels in Japanese and Korean, gain regulatory approval for the artwork, print thousands of labels, recall products from European depots, manually apply the new labels in a qualified facility, and redistribute everything across Asia.
By the time your first Japanese patient receives medication, your competitors have enrolled 200 patients in the same indication.
This scenario repeats daily across the pharmaceutical industry. Clinical supply leaders know that labeling represents one of the longest lead times and highest risk factors in bringing therapies to patients. Every country maintains distinct regulatory requirements for what must appear on investigational product labels, how information should be presented, and which languages are mandatory. These requirements conflict, overlap, and change with limited notice.
For those managing multi-region trials, the question becomes: how do you maintain compliance across 15 regulatory jurisdictions while preserving the agility to respond when protocols change, enrollment patterns shift, or new countries need to be added mid-study?
This article examines how Digital Display Labeling technology addresses constraints that paper-based systems cannot overcome. The approach has been validated across multiple pharmaceutical companies through regulatory submissions and real-world implementation.
What are the main challenges of managing country-specific label requirements?
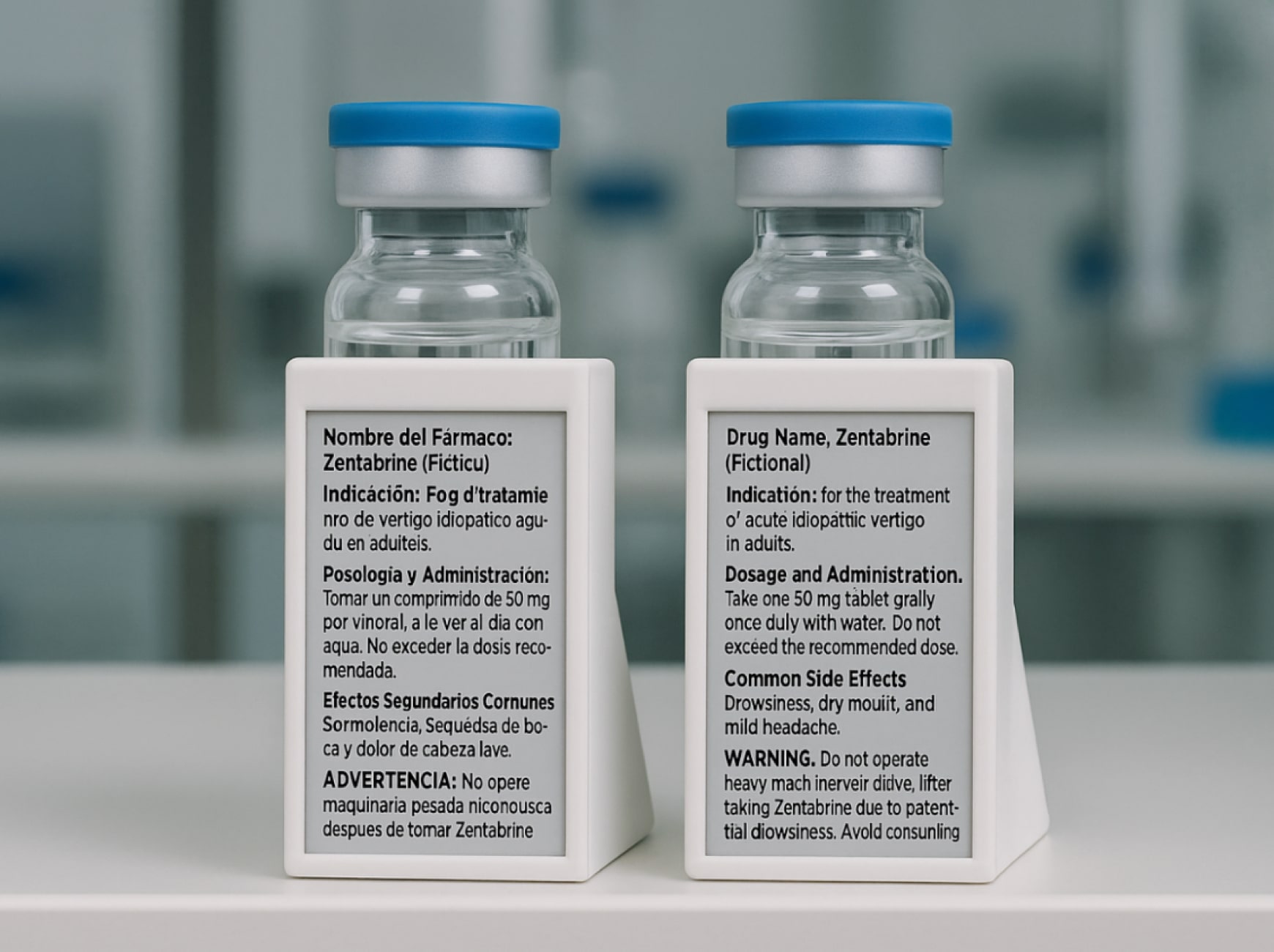
The regulatory landscape for clinical trial labels resembles a patchwork quilt with no consistent pattern. The United States requires specific cautionary language: “Caution: New Drug – Limited by Federal (or United States) law to investigational use.” Germany has its own requirements for batch number formatting and storage instruction phrasing. France mandates particular safety warnings. Japan requires specific symbols and text formatting that differs from other Asian markets.
A typical investigational medicinal product label contains up to 19 distinct regulatory elements. A trial spanning the United States, five European countries, three Asian markets, and two Latin American countries requires at minimum 11 distinct label versions. If your trial includes multiple dosage strengths or treatment arms, the number rises quickly to 30 or 40 unique labels. Each version needs its own artwork approval, printing batch setup, quality control verification, and inventory tracking system.
Most clinical supply operations need 4 weeks minimum between finalizing label content and completing packaging operations. When Germany’s health authority requests modifications to your storage instructions three weeks into the approval process, you either delay packaging by another month or proceed with other countries and circle back to Germany later, accepting the enrollment delay.
Paper labels create physical constraints. Vials and syringes offer minimal surface area. To fit all required information, manufacturers reduce font sizes to dimensions that challenge anyone over age 40 to read without magnification. The alternative involves booklet labels that fold out to reveal multiple panels of text, creating a poor user experience for clinical site staff.
One site coordinator in Frankfurt described the reality: “I have a patient waiting for discharge instructions, and I am flipping through six panels of a booklet label trying to find the dosing schedule. Half the text is in English, which I read but my patient does not. This takes time we do not have during a busy clinic session.”
Protocol changes expose the fundamental inflexibility of paper labels. When a stability study extends your product’s shelf life by six months, you face an immediate supply crisis. Products already labeled with the original expiry date cannot simply be updated. You must either scrap products approaching their labeled expiry date or initiate a relabeling program that consumes weeks and introduces quality risks at each manual step.
How can you efficiently manage multilingual pharmaceutical label translations and updates?
Translation work in clinical trials operates under constraints that do not exist in other industries. Clinical trial labels use technical terminology with specific meanings in regulatory contexts. The French term for “storage conditions” must match what France’s health authority expects to see. The German phrasing of “for clinical trial use only” must align with German regulatory conventions.
For a trial requiring ten languages, you are coordinating with ten different reviewer teams, each operating on their own timeline. A reviewer in Tokyo might return comments 3 weeks after receiving the draft. An expert in São Paulo might identify terminology issues that require consulting with the medical team.
Text length variations across languages create unexpected complications. French translations are typically longer than equivalent English text. If your label artwork was designed with tight spacing to fit all required information on a vial, the French version may not physically fit without reducing font size further or reimagining the entire layout.
Updates compound the problem exponentially. For a ten-language trial, you must update ten language versions, potentially requiring re-translation if the underlying meaning changed. Each language version follows its own approval pathway through your quality system and potentially through health authorities. Meanwhile, tracking which sites have which version of which language becomes an administrative burden.
Digital Display Labels change the fundamental equation by storing multiple complete language versions in the same device. The physical label remains constant while the displayed content adapts to the selected language. When you need to update dosing instructions, you modify the source text, complete translations for all required languages, and load the updated content to the DDL system.
Traditional multilingual label updates require weeks from approval to availability at sites. DDL content updates can be pushed to devices already in distribution within days of approval. A product sitting at a clinical site in Munich can receive updated content without leaving controlled storage.
Ensure clarity in every language, on every label
Reduce the risk of non-compliance and patient confusion from poor translations and unreadable text.
What is “supply pooling” and how does it simplify global inventory management?
Traditional clinical supply chains operate on a straightforward principle: manufacture products labeled for a specific protocol, ship them to sites enrolled in that protocol, and those products serve no other purpose. If Protocol X enrolls more slowly than forecasted while Protocol Y exceeds enrollment projections, you cannot simply reassign products from one protocol to another. This protocol-specific model creates significant inefficiency.
Supply pooling offers an alternative: manufacture investigational products without protocol-specific labeling. The problem is that paper labels make pooling operationally challenging. Assigning a pooled product to a specific protocol requires physically applying a protocol-specific label at a qualified facility.
Consider a realistic scenario: A site in Barcelona needs products for Protocol 302. Pooled inventory exists at a European depot 200 kilometers away. Under paper-based pooling, you must transport products from the depot to a labeling facility, apply Protocol 302 labels, document the labeling operation, and ship to Barcelona. This process requires one to two weeks.
Digital Display Labels make supply pooling practical by converting label assignment from a physical operation to a software operation. When the Barcelona site needs Protocol 302 products, the depot retrieves units from pooled inventory and updates the DDLs to display Protocol 302 content in minutes. The products ship directly to Barcelona without intermediate stops at labeling facilities.
When a trial experiences sudden enrollment acceleration, you can respond immediately by allocating additional units from pooled inventory. Traditional approaches require initiating new manufacturing campaigns that consume weeks. DDL-enabled pooling allows same-day allocation decisions followed by next-day shipping.
Can a single product SKU be used for multiple countries with different languages?
The traditional answer has been an unequivocal no. Each country’s distinct regulatory requirements necessitated unique SKUs. Your trial in Western Europe alone might require separate SKUs for Germany, France, Spain, Italy, Netherlands, Belgium, Switzerland, and Austria.
This SKU proliferation creates practical problems. When Germany enrolls faster than France, you cannot redirect French-labeled products to German sites. Manufacturing efficiency suffers from SKU proliferation. Smaller batch sizes for each country-specific SKU increase per-unit costs.
Digital Display Labels store multiple complete language versions and regulatory content sets in the same device. The physical product remains identical across countries. The differentiation happens at the point of dispensing rather than the point of manufacturing. When a product reaches a clinical site in Germany, site staff activate the DDL and select German language with German-specific regulatory content. The same physical product going to a French site displays French content.
From a manufacturing perspective, you produce a single batch equipped with multilingual DDLs containing all approved language versions for your trial. These products can be allocated to any participating country based on actual enrollment patterns rather than pre-committed forecasts.
Validation protocols demonstrate that when a product is assigned to Germany, the DDL displays German language content with German regulatory statements, not French or Spanish content. The system includes checks and controls that prevent misassignment. An audit trail documents every assignment: which product received which language and content set, when the assignment occurred, and who authorized it.
How do DDLs allow for on-demand language customization at the clinical site?
Language barriers in clinical trials create more than inconvenience. They introduce safety risks when patients cannot fully understand dosing instructions, storage requirements, or safety warnings.
A site in Toronto illustrates the challenge. The metropolitan area is highly multilingual, with substantial populations speaking English, French, Mandarin, Cantonese, Italian, Portuguese, and Punjabi. A clinical trial in oncology wants to enroll a diverse patient population representative of Toronto’s demographics. Paper labels can reasonably include English and French. Adding five more languages creates a label so large and complex that it becomes unusable.
The site coordinator faces an uncomfortable decision: restrict enrollment to English and French speakers, accepting reduced diversity, or enroll patients who speak other languages and provide informal translation assistance that may not accurately convey technical medical information.
Digital Display Labels store complete label content in as many languages as the trial supports. When a Mandarin-speaking patient enrolls, the site coordinator selects Mandarin from the available languages. The DDL displays all label information in Mandarin: storage instructions, dosing schedule, safety warnings, handling precautions, and any other required content.
This matters particularly for therapies that patients self-administer at home. A patient managing their own injections needs to clearly understand storage temperature requirements, injection timing, and what to do if a dose is missed. When caregivers are managing medication for family members, language access becomes even more critical.
Sites in multilingual regions no longer need to stock separate inventory for each language. A single product with a multilingual DDL can serve patients regardless of language preference. The DDL system logs which language was selected for each product when it was dispensed, creating an audit trail that is often more complete and reliable than manual logs.
Our DDLs let you achieve both diversity and clarity without compromise
Stop making the uncomfortable choice between limiting enrollment and risking patient safety.
How do DDLs help manage last-minute changes to study protocols?
Protocol amendments represent existential threats to supply chain timelines. When the medical team determines that dosing instructions need clarification, or when a safety committee recommends additional storage precautions, the supply chain impact cascades rapidly.
Consider a stability study scenario that occurs with frustrating regularity. Your initial stability data supported a 12-month expiry date for a clinical product. You manufactured and labeled 5,000 units with that expiry date. Six months into distribution, extended stability data demonstrates the product remains stable for 18 months.
Products approaching their labeled 12-month expiry cannot simply be used beyond that date, even though actual product stability supports it. Traditional relabeling requires coordinating with every clinical site and depot holding affected products. Products must be pulled from storage, transported to a qualified facility, physically relabeled with updated expiry dates, documented through your quality system, and redistributed. This operation consumes four to eight weeks and costs between $20 to $50 per unit.
Digital Display Labels convert this scenario from a weeks-long crisis to a days-long software update. When stability data supports an expiry extension, you update the content in the DDL system to reflect the new expiry date. The update is pushed to all affected devices, whether they are at manufacturing facilities, distribution depots, or clinical sites. Products remain in their current locations throughout the process.
Extended expiry dates allow continued use of existing inventory rather than forcing emergency manufacturing of replacement products. Emergency manufacturing carries premium costs and compresses timelines, often requiring 24-hour operations and expedited shipping. Avoiding emergency manufacturing saves hundreds of thousands of dollars per incident.
Dose-escalation studies benefit particularly from this flexibility. These trials intentionally modify dosing based on emerging safety data. Under paper-based systems, each dose modification requires new label artwork, printing, and application. DDL-enabled trials update displayed dosing information as protocols evolve, maintaining single inventory that displays current dosing guidance.
What is the process for adding a new country/language to a trial mid-study with DDLs?
Adding countries to ongoing trials exposes one of clinical development’s most frustrating inefficiencies. Your trial demonstrates promising efficacy in early results. Competitive intelligence suggests rivals are enrolling quickly in markets where you lack presence. You identify sites in South Korea ready to activate immediately. Everything aligns except one factor: labels.
Traditional label development for a new country follows a depressingly sequential process: develop label content meeting South Korean regulatory requirements, translate content into Korean, format translated content into label artwork, gain regulatory approval, print labels, ship labels to your manufacturing facility, apply labels to products, and ship labeled products to Korean sites.
This sequence consumes MONTHS under normal circumstances. By the time your first Korean patient enrolls, significant competitive ground has been lost.
Digital Display Labels eliminate most of this sequence. You still need to develop Korean-language content meeting Korean requirements and gain health authority approval. This work consumes six to eight weeks. Once Korean content receives approval, it is formatted for DDL display and loaded into the system as a new language option.
Products already manufactured with multilingual DDLs can immediately display Korean content. If suitable products exist in regional distribution in Asia, they can be redirected to Korea and configured upon arrival to show Korean language and regulatory content.
This speed advantage often determines whether adding a country mid-study proves worthwhile. If adding a country requires five months, enrollment might complete before the country contributes significant patient numbers. If adding a country requires two months, meaningful enrollment from that country becomes feasible.
Digital Display Labeling Constraints & Considerations
Every clinical supply leader reading this is thinking the same question: what about the hardware? Attaching electronics to pharmaceutical products that spend months in cold storage and ship across continents introduces legitimate technical challenges that need addressing.
Battery Life and Power Management
Current DDL prototypes use batteries, which raises sustainability concerns and introduces temperature limitations. Electronics perform poorly at extreme cold, making -80°C storage conditions for certain biologics and cell therapies problematic with current designs.
The way out is simple, implement DDLs first for room temperature and refrigerated products where the technology already performs reliably. Development work on energy harvesting, ultra-low-power displays, and rechargeable devices continues for ultra-cold chain applications. Companies are sequencing their DDL adoption to match current technical capabilities rather than waiting for perfect solutions across all temperature ranges.
Display Durability and Failure Rates
Electronic displays introduce different failure modes than paper labels. A malfunctioning display could show incorrect information rather than simply becoming unreadable. Current rigid electronics do not conform well to irregularly shaped containers and face mechanical stress during international shipping.
In order to battle that, we start with straightforward packaging formats like bottles and cartons where attachment is simple and mechanical stress is lower. Establish clear procedures for visual confirmation that displays are functioning correctly before dispensing. Develop backup processes for handling device failures, such as maintaining paper label capability as a fallback during early implementation phases. As field experience accumulates and designs improve, expand to more complex packaging formats.
Validation Requirements Beyond Software
Hardware-software integration requires qualification protocols that address scenarios unique to electronic labels: power loss during updates, interrupted wireless communication, and display integrity through temperature cycling. The validation burden extends to manufacturing facilities and clinical sites, requiring new procedures and training.
We use existing 21 CFR Part 11 compliance frameworks and extend them to cover hardware integration points. Develop standardized validation protocols that can be adapted across different DDL implementations rather than creating unique validation approaches for each trial. Pilot implementations in controlled settings allow companies to establish validation procedures before scaling to broader deployment.
Cost Economics
A paper label costs cents while a DDL costs dollars to tens of dollars. This difference matters for high-volume applications, making economic justification dependent on extracting value from dynamic labeling capabilities.
The practical approach: target DDL implementation at trials where value clearly exceeds cost. Adaptive trials, dose-escalation studies, and protocols with high likelihood of amendments and country additions justify the premium through avoided relabeling costs and reduced waste. For routine trials with stable protocols, paper labels remain economically sensible. Companies implementing reuse programs are establishing collection and refurbishment processes that reduce per-use costs over time, improving economics as operational experience grows.
Implementation Reality
Clinical supply leaders should view DDL technology as applicable to specific high-value use cases rather than universal replacement for paper labels. Current implementations focus on trials where supply pooling provides significant inventory reduction, protocol amendments are likely, or multilingual flexibility improves patient access. These targeted applications allow the industry to develop best practices and mature the technology before broader deployment. As costs decline and reliability improves through manufacturing scale and design iteration, the range of economically justified applications will expand.
Yalantis helps overcome these bottlenecks. We provide a fully managed transition service, from hardware selection and qualification to the validation of the end-to-end software process. This ensures your new paperless system is 21 CFR Part 11 compliant and audit-proof from day one, drastically reducing labeling-related deviations, improving operational flexibility, and creating a more robust, modern, and defensible supply chain.
Concerned about the hardware challenges?
Let’s design a controlled pilot to validate the technology on your products and establish best practices before you scale.
Conclusion
Digital Display Labels represent a fundamental rethinking of how clinical supply chains manage information. The technology addresses real constraints that have limited trial agility, increased waste, and delayed patient access to therapies for decades. But DDLs are not a universal solution. They introduce hardware challenges, validation complexity, and cost considerations that make them most appropriate for specific use cases where flexibility justifies investment.
The companies implementing DDLs today are doing so strategically: for trials with high amendment likelihood, therapeutic areas where supply pooling provides significant inventory reduction, or patient populations where multilingual flexibility improves diversity and access. These targeted implementations allow the industry to gain experience, develop best practices, and refine the technology before broader deployment.
As DDL technology matures, battery life will improve, costs will decline, and durability will increase. Regulatory pathways will become clearer as health authorities review implementations and provide guidance. What exists today represents early-stage technology with specific applications where value exceeds cost. What will exist in five years will likely expand those applications considerably as technical challenges are addressed and operational experience accumulates.
For clinical supply leaders, the question is not whether DDLs will become standard practice, but when and under what circumstances they make sense for your trials. Understanding both the capabilities and constraints positions you to make that decision strategically rather than reactively.