In the US, all medical facilities, doctors, nurses, and other so-called “covered entities” as well as everyone who works directly or indirectly with those entities (so-called “business associates”) have to comply with the Health Insurance Portability and Accountability Act (HIPAA) Security Rule for handling protected health information (PHI). The purpose of the Security Rule is to avoid breaches of patient data, and non-compliance can result in stiff fines. The HIPAA Security Rule obliges health service providers to implement the following safeguards in their organizations:
- Technical safeguards that impose healthcare software standards
- Physical safeguards that cover physical access to ePHI
- Administrative safeguards that entail organizational data protection measures
Maintaining compliance is critical, as non-compliance may lead to large penalties and harm a company’s reputation. For instance, in 2020, the Washington state-based health insurance company Premera Blue Cross paid $6.8 million for leaking ePHI of 10.4 million individuals.
Each country has its own legislation to ensure healthcare data privacy. In this post, we’ll compare HIPAA with health data security rules in Canada, the UK, Australia, the United Arab Emirates (UAE), the Kingdom of Saudi Arabia (KSA), and Qatar. If you want to enter these markets with your healthcare software, our article will be especially relevant for you.
Is HIPAA international or are there country-specific laws?
HIPAA is a U.S. federal law — and yes, it applies only in the US. If you’re asking, “Is there HIPAA in Canada?” or “Do other countries have HIPAA laws?” — the short answer is no. However, many countries have equivalent regulations. Each country has its own data privacy framework, and many follow similar principles: protecting personal health information, regulating third-party access, and enforcing penalties for misuse.
Here’s a side-by-side look at how HIPAA compares with laws in Canada, the UK, Australia, and MENA (Middle East and North Africa).

Now, we’ll discuss each country in detail. Let’s begin with health data standards in Canada.
Compliance mistakes cost more than you think
Cut rework and service costs before they happen with our free ISO 13485 + FDA compliance guide.
Does Canada have HIPAA?
So, does HIPAA apply in Canada? Not exactly. In Canada, the Personal Information Protection and Electronic Documents Act (PIPEDA) regulates private sector entities that collect, use, or disclose personal information for commercial purposes. These entities might include physicians who work in private practices and get paid directly by their patients or are reimbursed by patients’ provincial health plans or employment insurance plans.
PIPEDA pertains not only to medical data but also to such personal information as age, name, ID numbers, income level, ethnicity, credit records, loan records, and social status. Fines for breaking PIPEDA can be up to CAD$100,000 (~US$82,500).
|
Key Difference: Hipaa vs. Pipeda HIPAA only applies to health data. PIPEDA covers all personal data used in commercial settings — not just medical information. So if you’re handling financial, demographic, or employment data in Canada, PIPEDA applies — even outside of healthcare. |
In contrast to the HIPAA privacy rule, which applies to the whole US, PIPEDA does not apply to the provinces of Quebec, British Columbia, and Alberta. For example, private businesses in Quebec have to comply with the Quebec Act Respecting the Protection of Personal Information in the Private Sector (the Privacy Act 1993). Its principles and penalties are mostly in line with PIPEDA’s, though the Quebec Privacy Act relates to all private sector organizations, not only to those engaged in commercial activities.
That’s why before considering Canada as a new business landscape, take into account the provinces your business will operate in and their specific privacy laws. For instance, Ontario has additional legislation (apart from PIPEDA) called the Personal Health Information Protection Act (PHIPA) that covers only PHI. Penalties for non-compliance with PHIPA for individuals can be up to CAD$200,000 (~US$165,000) and for healthcare organizations can be up to CAD$1 million (~US$826,000).
Similarly to HIPAA, PHIPA regulates health information custodians (HICs) and HIC’s agents. HICs are equivalent to HIPAA’s covered entities, and HIC’s agents are similar to HIPAA’s business associates. At its core, PHIPA is largely like HIPAA.
One major difference is how consent works. Under HIPAA, healthcare providers can use patient data for treatment, billing, or internal operations without getting consent. In Canada, the default is the opposite. PHIPA usually requires patient consent — either implied or written — before collecting, using, or sharing health data.
Enforcement is also less centralized. HIPAA is backed by a federal agency that regularly investigates violations and issues large fines. Canada’s federal privacy commissioner can’t issue fines under PIPEDA. That may change soon. A new law, Bill C-27, would replace PIPEDA with a stronger framework — the Consumer Privacy Protection Act (CPPA). If passed, it would allow fines up to CAD$10 million or 3% of global revenue. Ontario’s PHIPA already has more teeth. The provincial commissioner can issue binding orders and refer cases to court.
Just like HIPAA, PIPEDA and other privacy laws in Canada’s provinces don’t limit organizations in the measures they take to ensure the security of patients’ data as long as these measures protect patients’ data lawfully and properly.
Is HIPAA applicable in the UK?
In the UK, protecting health data is more complicated and demanding than in the US. Healthcare organizations must comply with lots of acts, complete assessments, and adhere to standards. The UK government has developed a wide range of in-depth guides and policies to ease these procedures for healthcare businesses.
The UK’s data privacy framework is based on the UK GDPR, supported by the Data Protection Act 2018 (DPA 2018). Together, these laws govern how personal and health data must be handled. DPA 2018 sets the rules for how UK GDPR is applied and enforced within the country.
UK GDPR includes overall requirements for the protection of personal information, including health data. Specifically:
- Article 6 outlines the conditions organizations must meet to process personal data.
- Article 9 sets out when it’s lawful to process special categories of data, such as health information.
In the UK, the National Health Service (NHS) regulates not only how organizations handle PHI but also digital health products. Before launching in the UK market, all healthcare software solutions must:
- Be registered in the NHS Apps Library
- Successfully pass an NHS health app assessment
- Meet NHS digital, data, and technology standards
Сheck out the below guide to good practice for digital and data-driven health technologies to learn how to comply with all of the abovementioned requirements and ensure a proper level of health data privacy.

The UK’s data privacy laws are enforced by the Information Commissioner’s Office (ICO). The ICO can investigate complaints. It also reviews how organizations handle data and can step in when rules aren’t followed.
It also has the power to issue fines. For serious breaches, penalties can reach up to £17 million (~US$24 million) or 4% of a company’s global turnover.
Interested in healthcare product design & development services?
We develop medical devices, engineer healthcare software, and handle compliance from day one. By aligning hardware, firmware, cloud, and certification, we help you launch faster and reduce post-market costs up to 30%.
What’s the HIPAA equivalent in Australia?
In Australia, the main law regulating data privacy is the Privacy Act 1988. The essence of this act lies in the 13 Australian Privacy Principles (APPs). Some APPs deal specifically with PHI. Australian and Norfolk Island government agencies and most private sector organizations (collectively referred to as APP entities) must follow these principles when they handle personal information. Penalties for data breaches can be up to AU$2.1 million (US$1.6 million).
Compared to HIPAA, the Privacy Act 1988 covers a broader spectrum of personal information and regulates more entities. APP entities include:
- Organizations such as individuals (sole traders), corporate bodies, and partnerships
- Agencies such as ministries, federal courts, and the Australian Federal Police
- Businesses with an annual turnover of $3 million or more
- All private health service providers including private hospitals, pharmacists, gyms and weight loss clinics, and childcare centers
In Australia, health service providers have to follow all steps described in the Office of the Australian Information Commissioner’s Guide to health privacy. These steps are:
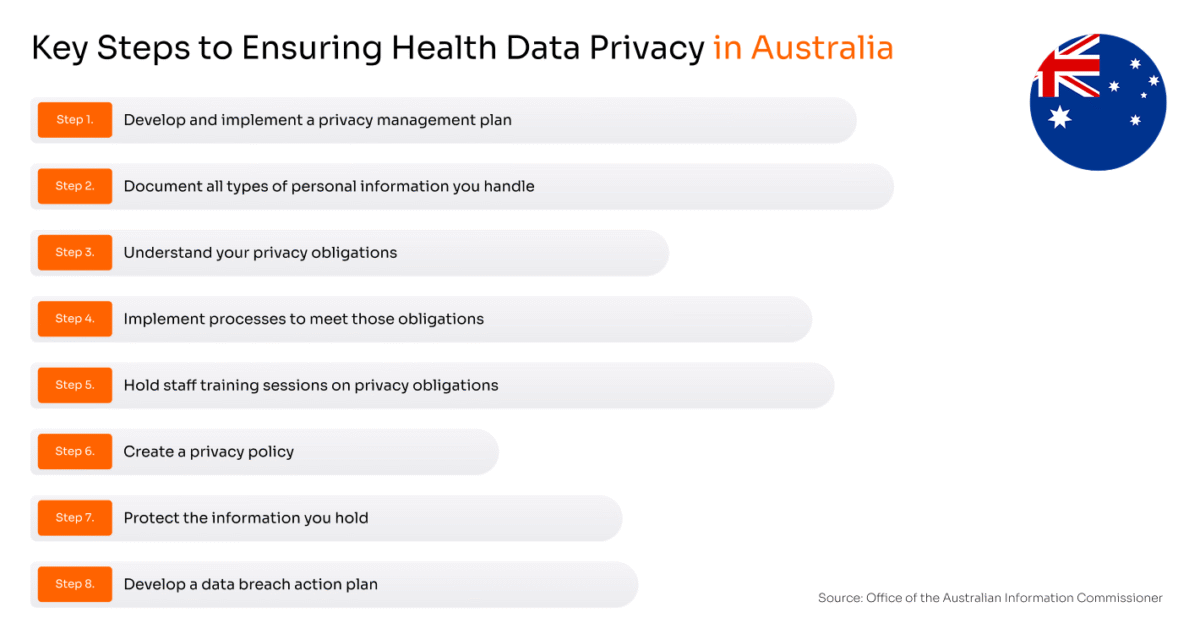
Healthcare is a prime target for attackers due to the value of medical records, and Australia’s strict reporting laws ensure incidents are swiftly exposed. This openness fosters accountability and drives stronger protections, but it also demands a responsible and mature approach to security from all organizations handling PHI in order to prevent breaches and avoid penalties.
— Dmytro Kravchuk, CISO at Yalantis
According to a report on the psychology of human error in the workplace, some of the most common causes of mistakes that lead to data breaches include:
- Falling for phishing emails. Such emails often look quite legitimate and seem to come from senior executives or respected brands.
- Sending emails to the wrong recipients. Distraction, stress, or fatigue in a fast-paced work environment are often the root cause of such errors.
How is PHI regulated in the MENA region?
According to APCO’s MENA Tech Trends report, MENA’s digital healthcare market is rapidly expanding. Countries in the MENA region are accommodating more and more technologies such as IoT, AI, machine learning services, and telemedicine.
In this section, we’ll outline the healthcare regulatory framework in the United Arab Emirates, the Kingdom of Saudi Arabia, and Qatar.
UAE: PDPL and the Health Data Law
The UAE has two key laws that apply to healthcare data.
First, there’s the Personal Data Protection Law (PDPL) — a federal law passed in 2021. It applies to most organizations that handle personal data, across all sectors. This includes health tech companies. The PDPL covers general privacy rights, rules for consent, cross-border data transfers, and the need to appoint a data protection officer in some cases. It’s the UAE’s closest equivalent to GDPR.
Second, there’s the Health Data Law, introduced in 2019. This law is specific to the healthcare sector. It covers hospitals, clinics, insurers, and any business that handles health records. It applies in both mainland UAE and free zones like Dubai, Abu Dhabi, and Sharjah.
Here are the key rules under the Health Data Law:
- Data use: Health data must only be used for clear, lawful purposes.
- Security: Providers must protect data with technical, operational, and organizational safeguards.
- Localization: You can’t transfer health data outside the UAE without approval from the Ministry of Health.
- Retention: Health records must be stored for 25 years after a patient’s last visit.
- Penalties: Fines range from AED 1,000 to AED 1 million (~US$272 to US$273,000).
|
What Else to Know Some zones have extra rules. For example, Dubai Healthcare City follows its own Health Data Protection Regulation (HDPR). It requires each organization to appoint a data protection officer. HDPR also sets clear rules on how to collect, store, and access health data. If you’re launching a healthcare product in the UAE, you’ll likely need to comply with both PDPL and the Health Data Law. One governs personal data in general. The other focuses on medical records. Together, they shape how healthcare data must be handled. |
Saudi Arabia: Personal Data Protection Law (2021)
Saudi Arabia passed its first general data privacy law — the Personal Data Protection Law (PDPL) — in 2021. It was updated in 2023 and is being rolled out gradually.
PDPL applies to any company that handles personal data inside Saudi Arabia, even if the company is based elsewhere. It defines the rules for collecting and using personal data. It also lays out when consent is needed and how to handle things like access requests or data leaks. Some companies must also appoint a local data protection officer.
Transferring data outside Saudi Arabia is restricted. You’ll need permission from the Saudi Data and AI Authority (SDAIA), which enforces the law.
If you’re working in healthcare, PDPL isn’t the only law to keep in mind. The sector also follows other rules, including:
- The Law of Practicing Healthcare Professions (PHP)
- The Private Health Institutions Law
- Saudi Health Information Exchange (SeHE) policies
These laws require patient consent before data is shared. They also set basic expectations for privacy. But they don’t cover everything. PDPL fills in the rest — especially around things like data access rights and international transfers.
If you’re building a health product for the Saudi market, you’ll need to follow both sets of rules.
Qatar: Data Protection Law No. 13 of 2016
In Qatar, data privacy in all sectors including healthcare is regulated by the Personal Data Privacy Protection Law (PDPPL) of 2016.
The single authority in charge of PDPPL is the Compliance and Data Protection (CDP) department. Fines for non-compliance can range from QAR 1 million to 5 million (~US$275,000 to US$1.3 million).
PDPPL requires organizations handling personal data to follow clear data protection practices, comparable in some aspects to HIPAA. This includes:
- Administrative measures like employee training and internal privacy policies
- Technical safeguards such as secure data storage and access control
- Organizational responsibilities to ensure consistent and lawful data handling
The law also sets conditions for consent, limits on international data transfers, and gives individuals the right to access and correct their data.
Final word
Most countries include health data requirements in their general legislation on data privacy, though not all highlight specific measures to protect this sensitive data. Some countries such as Australia and the UK provide detailed guidelines on how to interpret data protection acts as well as tips on how to comply in the best way. Countries from the MENA region are still changing and updating their laws following global digitalization trends.
Yalantis offers extensive expertise in building holistic healthcare solutions (as IoT healthcare solutions). We’ll gladly back you up in building a secure healthcare product.
FAQ
What is the HIPAA equivalent in Canada?
The HIPAA equivalent in Canada is the Personal Information Protection and Electronic Documents Act (PIPEDA). In healthcare, Ontario’s PHIPA also plays a key role.
What is the HIPAA equivalent in Europe?
The closest European equivalent of HIPAA is the General Data Protection Regulation (GDPR), which sets strict rules on processing health data.
What is the HIPAA equivalent in the UK?
The HIPAA equivalent in the UK is the UK GDPR, supported by the Data Protection Act 2018, which governs how health data must be handled.
Is HIPAA only in the US?
Yes, HIPAA is only in the US. Other countries, including Canada, the UK, Australia, and MENA states, have their own health privacy laws.
Can Yalantis help create HIPAA-compliant healthcare services?
Yes, Yalantis can design and develop secure healthcare solutions that comply with HIPAA as well as other international privacy regulations.








